前までは通っていた病院でアナフラニールを処方して頂いてましたが、こちらでも効き目は問題ありません。精神状態を安定してくれて、とても私生活が楽になります。お安く買えるのも経済的に助かるので、今後もこのサイトでお薬を購入し続けます。

左記クレジットカード、銀行振込、コンビニ決済に対応




更新日:2025/4/16

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 39円 | 3,960円 | 118pt | |
| 200錠 | 35円 | 7,160円 | 214pt |
| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 71円 | 7,160円 | 214pt | |
| 200錠 | 53円 | 10,760円 | 322pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。


うつ症状の改善
10mgx100錠
3,560円

うつ症状の改善
10mgx28錠
4,560円

うつ症状の改善
50mgx100錠
7,260円

うつ症状の改善
20mgx28錠
4,560円

うつ症状の改善
20mgx100錠
6,060円

うつ症状の改善
60mgx28錠
7,160円

うつ症状の改善
100mgx30錠
4,860円

うつ症状の改善
25mgx100錠
5,460円

うつ症状の改善
5mg28錠x1箱
4,860円

うつ症状の改善
20mg200錠x1箱
11,960円

うつ症状の改善
15mgx100錠
6,560円

うつ症状の改善
15mgx28錠
4,960円
クロフラニール 25mg x 100錠
3,960円
ポイント:118pt
10,000円以上購入で送料無料
在庫あり

前までは通っていた病院でアナフラニールを処方して頂いてましたが、こちらでも効き目は問題ありません。精神状態を安定してくれて、とても私生活が楽になります。お安く買えるのも経済的に助かるので、今後もこのサイトでお薬を購入し続けます。
飲み始めてから3日目。ちょっと副作用がきついかもしれない。もうちょっと飲んでも慣れそうにないなら、他のにしてみる。
うつ病治療薬の中には、適切な用法用量を守らず使用した場合には、依存してしまう可能性があるものもあります。適切に服用しなかった場合には副作用のリスクも高まってしまいます。薬を服用することで効果的にうつの改善を目指せるため、適切な用法用量を守ってご使用ください。
薬の服用で副作用が強くあらわれるといったような場合、継続して服用すれば副作用症状が緩和していくといったこともあります。ですが、あまりにも合わないといったような場合には、医師と相談しながら減薬などを進めていくといった必要があります。
薬を服用することによって、うつ病による不安などの症状を緩和することができます。その上で、適切なカウンセリングなどを受けることでうつ病を根本的に改善へと導けるようになります。
うつ病の薬の作用に生理が来なくなるといったものはありません。しかし、うつ病によるストレスや食欲の低下などがホルモンバランスに影響して生理が遅れたり、止まったりする可能性があります。
うつ病治療薬の中止や休薬は計画的に行う必要があります。そのため、無計画に休薬などをしてしまった場合、パニックなどの離脱症状があらわれる可能性があります。そのため、薬の服用を中止する場合には、事前に医師に相談するようにしてください。
| 1日の服用回数 | 1~3回 |
|---|---|
| 1回の服用量 | 17~100mg |
| 服用のタイミング | 指定なし |
| 服用間隔 | 6時間以上 |
| 1日の服用回数 | 1~2回 |
|---|---|
| 1回の服用量 | 10~50mg |
| 服用のタイミング | 指定なし |
| 服用間隔 | 6時間以上 |
| 1日の服用回数 | 1~3回 |
|---|---|
| 1回の服用量 | 3~75mg |
| 服用のタイミング | 指定なし |
| 服用間隔 | 6時間以上 |
| 商品名 | フルニル | ベネジスXR | シタロプラム | アロー | サミー | ダイロキシア | ブプラパン |
|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |
| 特徴1 | ・副作用や依存性が少ない | ・神経伝達物質に影響を与えて気分をコントロールする | ・効果の持続時間が長い | ・SSRI(SNRI)で効かない場合に有効な抗うつ薬 | ・もともと体内にある天然物質を配合 | ・不安をやわらげて、意欲を高める | ・アメリカでは広く流通している抗うつ剤 |
| 特徴2 | ・プロザックよりも安価で大容量 | ・国内の抗うつ薬と同成分を配合している | ・レクサプロ・ジェネリックなどの成分の元となった成分を配合 | ・作用が強いので高い改善効果を期待できる | ・優れた抗酸化作用にも期待することができる | ・糖尿病性神経障害による、しびれや痛みにも有効 | ・禁煙の成功も高める |
| 内容量 | 10mgx100錠 | 37.5mg28錠x1箱 | 20mgx28錠 | 10mgx100錠 | 200mgx30錠 | 30mg28錠x1箱 | 300mg30錠x1箱 |
| 価格 | 3,560円 | 3,560円 | 4,560円 | 3,260円 | 5,760円 | 4,660円 | 5,060円 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
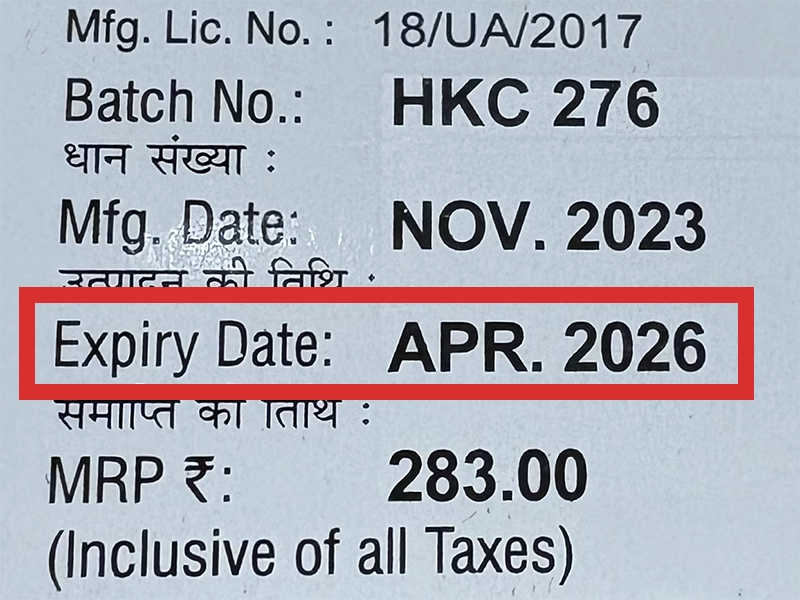
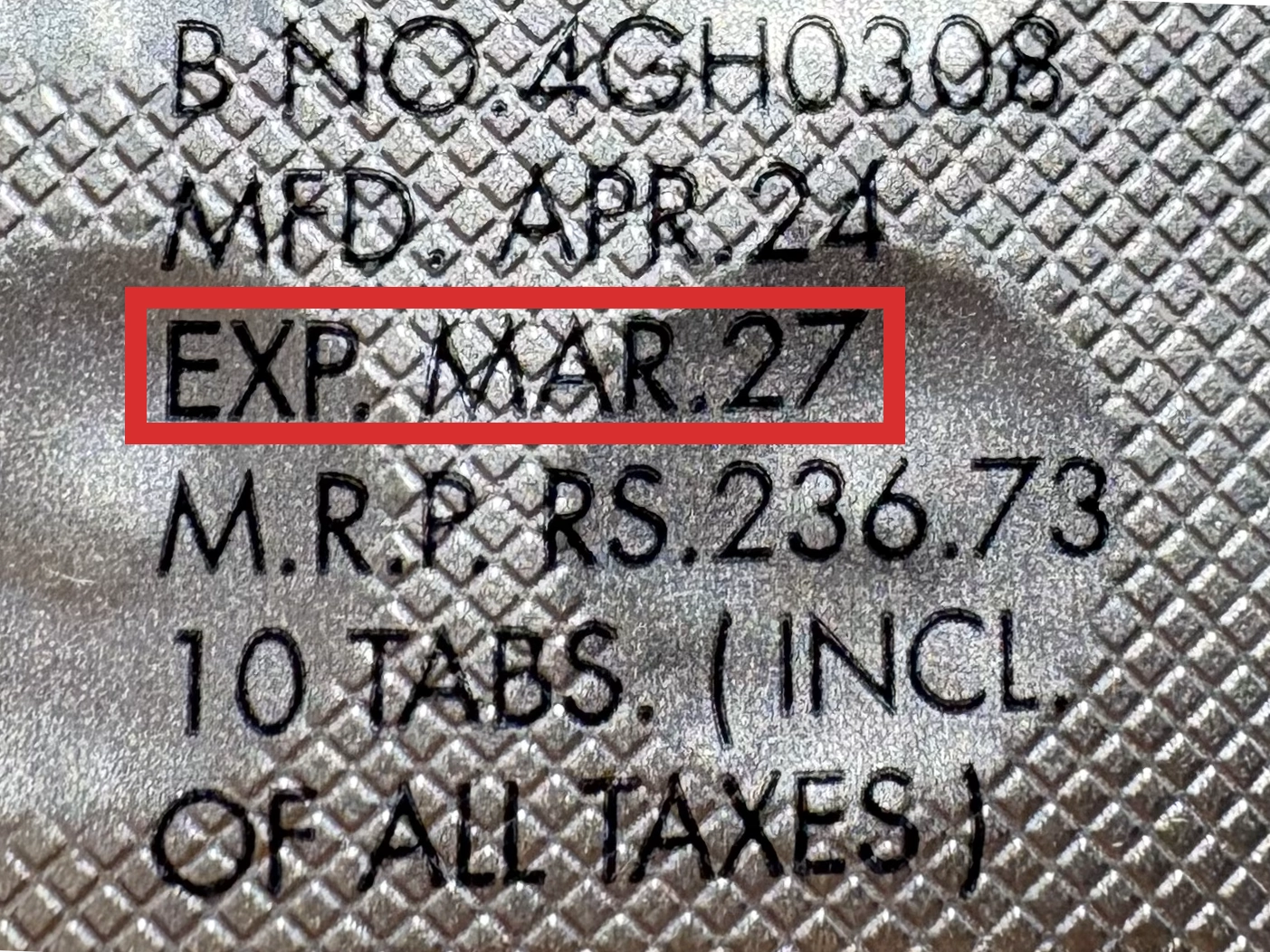
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
前までは通っていた病院でアナフラニールを処方して頂いてましたが、こちらでも効き目は問題ありません。精神状態を安定してくれて、とても私生活が楽になります。お安く買えるのも経済的に助かるので、今後もこのサイトでお薬を購入し続けます。
副作用は口が渇いてくるくらい。これくらいなら全然大丈夫。最近は意欲が沸いてくるし、考え方も前向きになってきたから、薬に頼ってみて良かった。
うつ状態で悩んでたけど、これを飲んでから落ち着いてきた。でも、仕事中に眠くなってくるから、なかなか仕事に集中できない。
飲み始めてから3日目。ちょっと副作用がきついかもしれない。もうちょっと飲んでも慣れそうにないなら、他のにしてみる。
私にはよく効いてくれますし、錠数が多いのに安く買るなんて本当に助かります。これからもお世話になります。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。