家を出てしばらくしてから鍵閉めた?とか、エアコン切った?とか、ガスの元栓閉めた?とか気になって、わざわざ家に戻って確かめることが多くなり悩んでました。どうやら強迫性障害らしいってことでフルニル飲むようになりました。20日間ほど飲み続けて、不安が和らいでる気がしてます。

左記クレジットカード、銀行振込、コンビニ決済に対応





更新日:2025/4/8

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 35円 | 3,560円 | 106pt | |
| 200錠 | 25円 | 5,060円 | 151pt | |
| 300錠 | 21円 | 6,360円 | 190pt |
| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 41円 | 4,160円 | 124pt | |
| 200錠 | 36円 | 7,360円 | 220pt | |
| 300錠 | 32円 | 9,760円 | 292pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。


うつ症状の改善
10mgx28錠
4,560円

うつ症状の改善
50mgx100錠
7,260円

うつ症状の改善
25mgx100錠
3,960円

うつ症状の改善
20mgx28錠
4,560円

うつ症状の改善
20mgx100錠
6,060円

うつ症状の改善
60mgx28錠
7,160円

うつ症状の改善
100mgx30錠
4,860円

うつ症状の改善
25mgx100錠
5,460円

うつ症状の改善
5mg28錠x1箱
4,860円

うつ症状の改善
20mg200錠x1箱
11,960円

うつ症状の改善
15mgx100錠
6,560円

うつ症状の改善
15mgx28錠
4,960円
フルニル 10mg x 100錠
3,560円
ポイント:106pt
10,000円以上購入で送料無料
在庫あり

家を出てしばらくしてから鍵閉めた?とか、エアコン切った?とか、ガスの元栓閉めた?とか気になって、わざわざ家に戻って確かめることが多くなり悩んでました。どうやら強迫性障害らしいってことでフルニル飲むようになりました。20日間ほど飲み続けて、不安が和らいでる気がしてます。
フルニルを飲み始めてから、日中にうとうとすることが多くなった。仕事に支障が出そうで心配。
うつ病、強迫性障害、パニック障害、過食症などに使われます。これらは気分の落ち込みや強い不安、食行動の乱れなどがある症状ですがフルニルが脳内のセロトニンに働きかけて症状の改善を促します。
フルニルは、うつ病による長期間の気分の落ち込みに効果がある薬です。多くの患者さんで、継続して飲むことで気分が少しずつ改善されていくことが確認されています。効果が出るまでには数週間かかることもあるので、焦らずに続けることが大切です。
うつ病の急性期・維持期の治療において広く効果が確認されています。特に20mgの用量でも十分な改善が得られるケースが多く、症状の再発防止にも有効です。高齢者にも効果が認められています。
フルニルは広場恐怖を伴うパニック障害にも効果があります。人混みや外出に対する強い不安感やパニック発作を和らげる作用があり、外出しやすくなる方もいます。継続して飲むことで、生活の幅が広がる可能性があります。
朝に服用することが指示されています。これは一部の人に不眠の副作用が出ることがあるためで、朝の服用の方が体への負担が少ないとされています。夜勤などで朝の服用が難しいといった場合は、医師に相談した上で適切なタイミングで服用してください。
最初は少ない量から始めて、様子を見て徐々に増やしていくのが一般的です。急に増やすと副作用が出やすくなるため、慎重に段階的に調整されます。医師の指示通りに飲むのが安心です。
フルニルは毎日同じ時間に1回服用することが基本です。飲み続けることで血中濃度が安定し、効果も持続します。自己判断で休薬したりせず、指示通りに継続することが重要です。
飲み忘れに気づいたら、できるだけ早くその日のうちに飲んでください。ただし、次の服用時間が近い場合は、その回は飛ばして通常通りに戻すことが勧められます。2回分を一度に飲むのは避けましょう。
不眠やイライラといった症状が副作用としてあらわれることがあります。とくに飲み始めの時期や増量後に多く見られる傾向があります。気になる症状が続く場合は、無理せず医師に相談してください。
飲み始めは副作用が出やすい時期なので不眠や不安感、吐き気などに注意が必要です。これらは時間とともに軽減していくことが多いですが、強く現れる場合は医師に相談しましょう。
フルオキセチンを急にやめると不安感やめまい、頭痛や電気ショックのような感覚などの離脱症状が出ることがあります。やめるときは必ず医師の指導のもとで、少しずつ減らしていくことが大切です。
フルニルは注意力や判断力に影響を及ぼすことがあるため、車の運転や危険を伴う作業は控えた方が良いとされています。とくに副作用が出やすいとされている服用初期や増量時には注意が必要となります。
| 1日の服用回数 | 1~2回 |
|---|---|
| 1回の服用量 | 20~80mg |
| 服用のタイミング | 朝のみもしくは、朝と正午 |
| 服用間隔 | 6時間以上 |
| 商品名 | インシドン | アゴプレックス | スティリザン | セルタファイン | アロー | デピロックス | セントジョーンズワート | セドキシル |
|---|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |  |
| 特徴1 | ・神経伝達物質のバランスを整える | ・睡眠障害にも効果が期待できる | ・不安や抑うつ症状、統合失調症などに効果的 | ・憂うつな気分や不安感をやわらげる | ・SSRI(SNRI)で効かない場合に有効な抗うつ薬 | ・アモキサンより安価に購入できる | ・軽度から中等度のうつ病の治療に有効 | ・慢性胃炎や心臓神経症にも効果が期待できる |
| 特徴2 | ・古くから使用される三環系抗うつ剤 | ・従来品よりもさらに副作用の心配が少ない | ・他の医薬品にも使われている成分なので信頼できる | ・従来の抗うつ薬と比べて、口渇や便秘などの副作用が軽減されている | ・作用が強いので高い改善効果を期待できる | ・比較的効果が早く現れる | ・うつ病に伴う不眠症の改善にも期待できる | ・筋肉をほぐして緊張型頭痛にも効果を発揮 |
| 内容量 | 50mg100錠x1箱 | 25mg50錠x1箱 | 1mg30錠x1箱 | 100mg100錠x1箱 | 10mgx100錠 | 50mgx100錠 | 375mg120錠x1本 | 1mg60錠x1箱 |
| 価格 | 9,160円 | 6,560円 | 3,860円 | 6,260円 | 3,260円 | 4,660円 | 4,260円 | 6,560円 |
| 頻度が高いもの | 頻度が低いもの | 稀なもの | |
| 身体全体 | 悪寒 | 自殺未遂 | 急性腹部症候群、光線過敏症反応 |
| 心臓血管系 | 動悸 | 不整脈、低血圧 | |
| 消化器系 | 嚥下困難、胃炎、胃腸炎、下血、胃潰瘍 | 血性下痢、十二指腸潰瘍、食道潰瘍、胃腸出血、吐血、肝炎、消化性潰瘍、胃潰瘍出血 | |
| 血液およびリンパ系 | 斑状出血、稀:点状出血、紫斑 | ||
| 検査 | QT間隔の延長(QT c F ≥450ミリ秒) | ||
| 神経系 | 情緒不安定 | アカシジア、運動失調、平衡障害1、歯ぎしり1、頬舌症候群、離人感、多幸感、筋緊張亢進、性欲亢進、ミオクローヌス、妄想性反応、妄想 | |
| 呼吸器系 | 喉頭浮腫 | ||
| 皮膚および付属器 | 脱毛症、紫斑 | ||
| 特殊感覚 | 味覚異常 | 散瞳 | |
| 泌尿生殖器系 | 排尿障害 | 排尿困難、婦人科出血 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
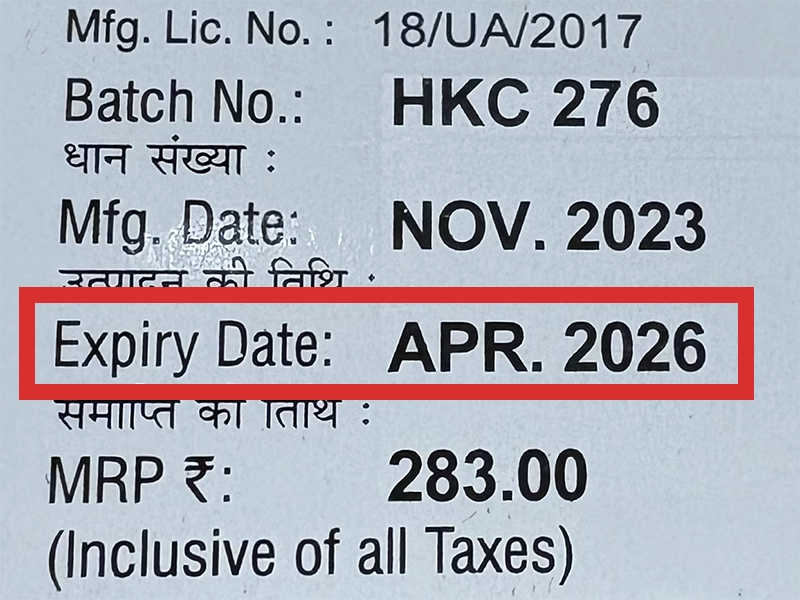
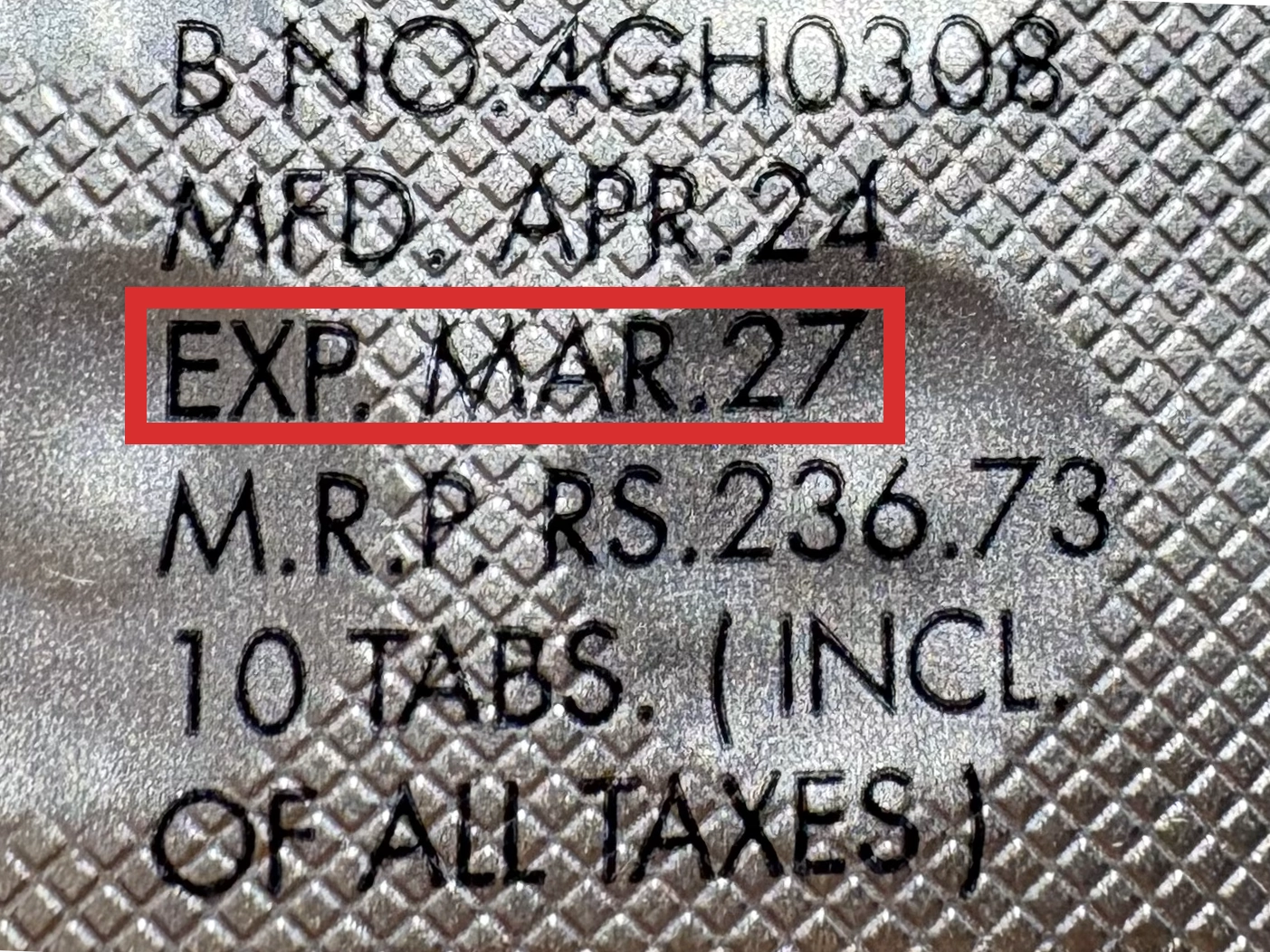
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
プロザック飲んでたけど、値段がより安いジェネリックのフルニルに乗り換えました。プロザックと効果は変わらないと思います。
写真で見て思ってたイメージよりも、あまり綺麗でない箱の商品が届いて来て、大丈夫かな?て少し不安になりました。半信半疑ながら飲み続けて2週間過ぎた頃に気分が落ち着いてきたような気がします。
依存性のない薬を探しててフルニル試してみました。プロザックのジェネリックなので全然安いです。ただ、強い薬を飲んでた時と比べると、効いてるか効いてないのかわかりにくいですね。飲み続けないといけないってことだけど。
家を出てしばらくしてから鍵閉めた?とか、エアコン切った?とか、ガスの元栓閉めた?とか気になって、わざわざ家に戻って確かめることが多くなり悩んでました。どうやら強迫性障害らしいってことでフルニル飲むようになりました。20日間ほど飲み続けて、不安が和らいでる気がしてます。
以前はそうでなかったのに、上司に叱責されてから、人前で話すのが怖くなることが多くなり、気分を落ち着けるためにフルニル飲んでます。すぐ効くタイプの薬じゃないので、飲み続けることで効果を感じれています。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。