苦みがほとんどなく、翌日に口の中に苦みが残ることもありませんので、不快感なく使用できます。急激に眠くなるのではなく緩やかに眠くなってくるため、本当に自然な感じで眠ることができました。環境が大きく変化したためか、引っ越してからずっと不眠症が続いているため、不眠症を完全に乗り越えるまで続けていくつもりです。

左記クレジットカード、銀行振込、コンビニ決済に対応


更新日:2025/6/23
ソミナーは、抗ヒスタミン作用によって眠気を引き起こす睡眠薬です。
従来の睡眠薬と作用の仕組みが異なるため、睡眠薬にありがちな副作用のリスクが低いという特徴があります。
寝つきが悪い入眠障害や、夜中に目が覚めてしまう中途覚醒に効果的です。
| メーカー | チャロエン・バエサジ・ラボ(Charoen Bhaesaj Lab) |
|---|---|
| 有効成分 | コハク酸ドキシラミン |
| 効果 | 不眠症の改善・アレルギー症状の緩和 |
| 副作用 | 傾眠や口の渇きなど |
| 用法 | 就寝直前に服用 |
コハク酸ドキシラミンには、脳の覚醒に深い関わりを持つヒスタミン(神経伝達物質)を抑制することで、スムーズな睡眠に導く作用があります。脳内においてヒスタミンH1受容体に結合することによって、その効果を発揮します。
ソミナーには、1錠あたり25mgのコハク酸ドキシラミンが配合されています。

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 200錠 | 36円 | 7,200円 | 216pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

ソミナー 25mg x 200錠
7,200円
ポイント:216pt
10,000円以上購入で送料無料
在庫あり

苦みがほとんどなく、翌日に口の中に苦みが残ることもありませんので、不快感なく使用できます。急激に眠くなるのではなく緩やかに眠くなってくるため、本当に自然な感じで眠ることができました。環境が大きく変化したためか、引っ越してからずっと不眠症が続いているため、不眠症を完全に乗り越えるまで続けていくつもりです。
飲み続けてたら効かなくなってきた。耐性がついたのかな。仕方がないから他の睡眠薬にする。
ソミナーは脳の働きをゆるやかにして、自然な眠気を促す作用があります。「なかなか寝つけない」「寝ようと思っても目が冴える」といった入眠の悩みに対して、睡眠導入を助ける市販薬として広く使用されています。抗ヒスタミン薬としての作用を利用し、一時的な不眠症状の改善に役立ちます。
多くの場合、服用してから30分ほどで眠気があらわれます。寝る少し前に飲むようにすると効果的です。服用して速やかに眠気が訪れるようになるので、用事などを済ませてから服用するようにしましょう。
ソミナーは主に入眠を助ける目的で作られており、中途覚醒を防ぐ作用も一部ありますが、夜中の覚醒が主な症状の場合は、そうしたタイプの不眠に有効な睡眠薬の使用が適しています。
他の睡眠薬との併用はお避け下さい。効果が強くなりすぎて副作用のリスクが上昇してしまいます。その結果、翌朝目覚めた後も眠気が残ってしまう可能性もあります。
ソミナーは就寝の30分前に1錠を服用します。夜間の睡眠をサポートする目的でのみ使用され、1日に1回を超えて飲まないようにしてください。眠気が続く可能性があるため、服用後は必ず横になれる状況で使うことが大切です。
いいえ、アルコールとの併用は避けるべきとされており、眠気や集中力の低下が強く出ることがあります。食後の制限はありませんが、空腹時の方が効果が早くあらわれる場合があります。
ソミナーは「一晩寝る時間が確保できるとき」にのみ使用すべきです。十分な睡眠時間が取れないと、翌日の眠気や注意力の低下につながるため注意してください。
多めに服用するのは厳禁です。効果が強くなるよりも副作用のリスクが高くなるため、多めに服用したりすることはせず、適切な用法用量を守ってご使用ください。
はい、ソミナーは作用が長く続くことがあり、翌朝も眠気やぼんやり感が残ることがあります。特に十分な睡眠時間を確保できないまま使用した場合、日中の集中力や反応速度に影響することがあるため、自動車の運転や機械の操作は避けるべきです。
はい、高齢者では薬の影響が強く出ることがあり、転倒や混乱を招くおそれがあります。年齢に応じて慎重に使用し、できるだけ短期間で使うことが推奨されます。
抗うつ薬や抗不安薬、アルコールなどと一緒に使うと、眠気や呼吸抑制が強くなることがあります。併用薬がある場合は必ず併用しても問題がないかを適切に把握した上で服用するようにしてください。
口の渇きや便秘、頭痛などが起こることがあります。まれに皮膚の発疹やアレルギー症状が出ることもあるため、気になる症状があればすぐに服用をやめて医療機関を受診するようにしてください。
| 1日の服用回数 | 1回 |
|---|---|
| 1回の服用量 | 25mg |
| 服用のタイミング | 就寝前 |
| 服用間隔 | 指定なし |
| 商品名 | フルナイト | ハイプロン | ハイプナイト | ソクナイト | アモバン・ジェネリック | メラトニン(NOW Foods) | ソナプロン | ドリエル・ジェネリック | APOゾピクロン |
|---|---|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |  | |
| 特徴1 | スムーズに入眠できる超短時間型の睡眠薬 | すみやかに入眠できる超短時間型 | 実績ある睡眠薬ルネスタのジェネリック | 超短時間(2~4時間)のみ効果を発揮 | 入眠障害に効くアモバンのジェネリック | 自然な入眠が期待できる「快眠サプリ」 | 即入眠できる有効成分ザレプロンを配合 | ・ドリエルと同じ成分を配合したジェネリック | ・夜なかなか眠れない方におすすめの睡眠薬 |
| 特徴2 | 作用時間が短く、翌朝に眠気が残りにくい | 寝覚めがよく眠気を引きずらない | 依存性が比較的低い有効成分を配合 | ふらつきや転倒などが起きにくい | 翌朝に眠気を引きずらない | 製造元は老舗サプリメーカーの「NOW Foods」 | 半減期が短いので翌朝に残らない | ・大容量で1錠8円と破格 | ・即効性が高く、依存性も低い |
| 内容量 | 2mgx50錠 | 10mgx100錠 | 1mgx50錠 | 2mg50錠x1箱 | 7.5mgx30錠 | 3mg60錠x1本 | 10mgx30錠 | 25mgx600錠 | 7.5mgx30錠 |
| 価格 | 4,110円 | 4,590円 | 3,000円 | 4,110円 | 2,400円 | 4,160円 | 1,800円 | 5,600円 | 3,100円 |
| 頻度不明 | |
| 精神神経系 | 眠気、頭痛、興奮 |
| 呼吸器 | 口、鼻、喉の乾燥 |
| 消化器 | 吐き気 |
| 感覚器 | 視覚異常 |
| その他 | 胸の締め付け、神経質、排尿困難 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
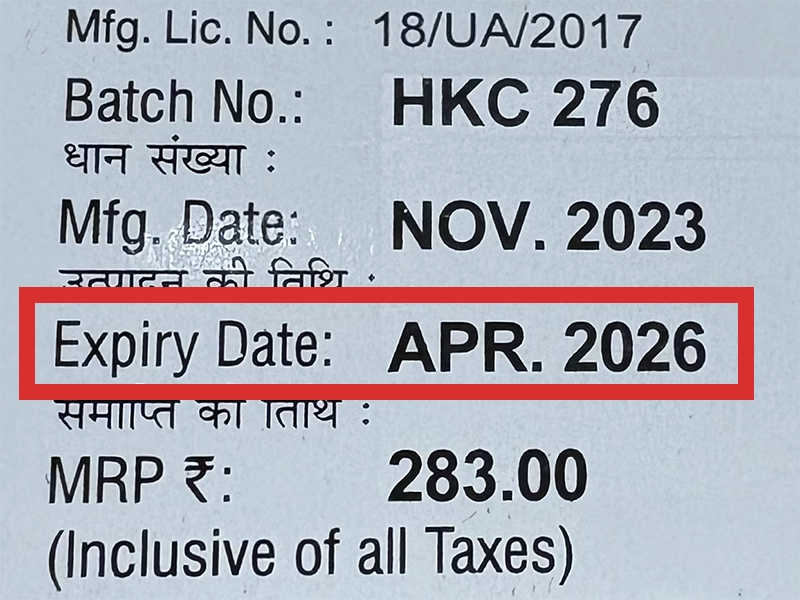
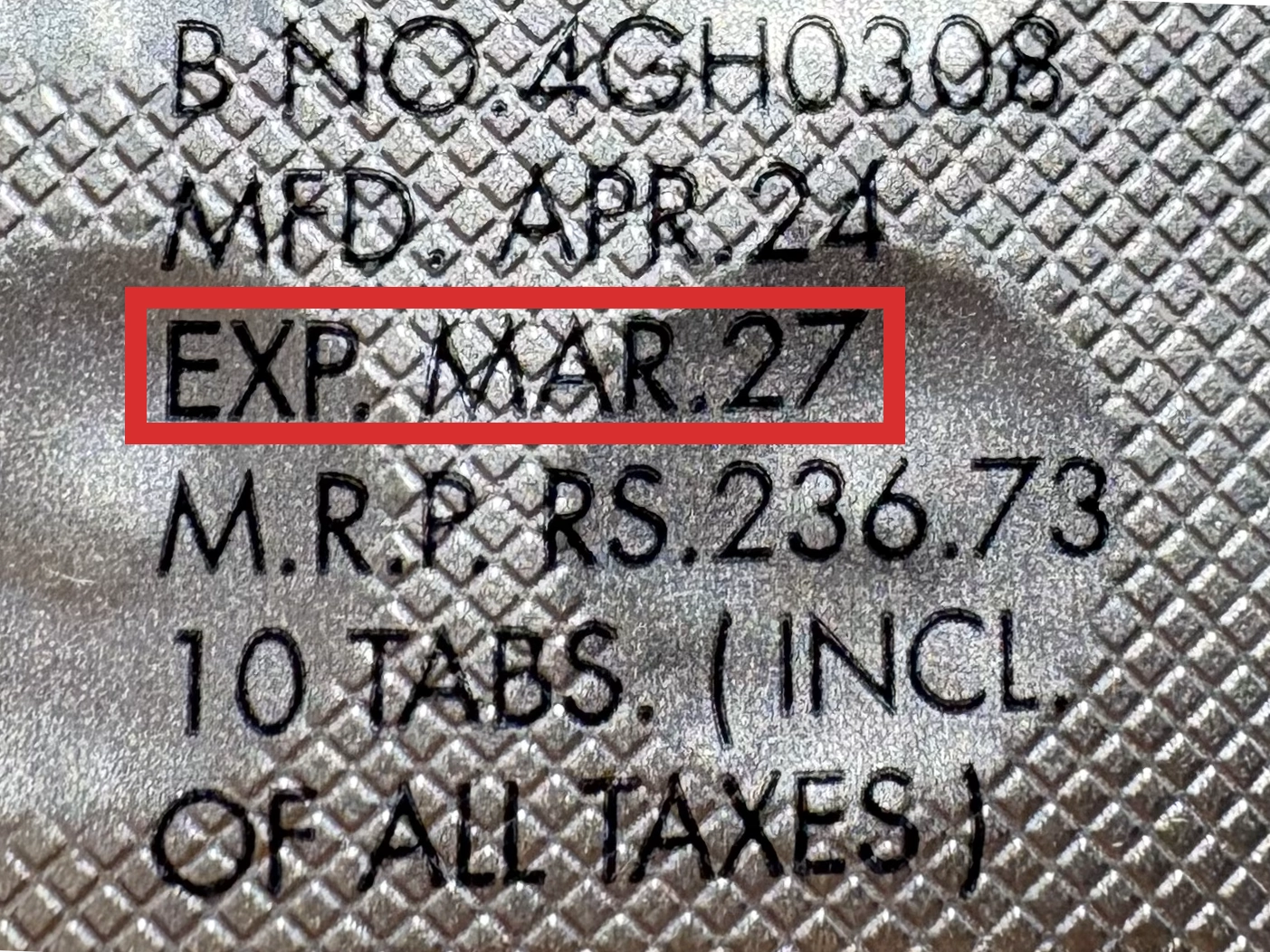
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
1錠だけでもしっかり眠くなる。眠くなり方も強烈に眠たくなって寝かしつけられるような感じではなく、自然と眠くなってきて寝たくなる感じ。でも、しばらく使っていたらだんだん効きが悪くなってきたから、今は2錠飲むようにしている。場合によっては耐性ができてしまうのかもしれない。入眠効果が高いからといって頼りにしすぎるのは注意したほうがいいかも。
翌日に全く響かないので、飲んでいて安心感があります。即効性はそこまでないように感じました。病院で出してもらっている睡眠薬の効きが悪いとき、かわりに飲むようにしていますが、即効性が高い薬でさっと眠りたいという人だと物足りなさを感じてしまうかもしれません。私はこれくらいの効き目でも別にいいかなと感じたので、これからもピンチヒッターとして利用します。
ぐっすり眠れますし、花粉の時期に飲むと花粉症の鼻詰まりが解消されて、寝るときの不快感がなくなりました。1錠では少し効きすぎましたが、半錠にするとちょうどいい効き具合でした。今のところは耐性がついている感覚もなく使えています!
クリニックで出される睡眠薬は副作用が強く、翌日にも眠気が残りやすく、とにかく使いにくさを感じていました。こちらの製品はまだ一度しか飲んでいませんが、そういったことがなく、次の日にすっきり目覚めることができました。使い勝手が良さそうなので、このまま続けます。
苦みがほとんどなく、翌日に口の中に苦みが残ることもありませんので、不快感なく使用できます。急激に眠くなるのではなく緩やかに眠くなってくるため、本当に自然な感じで眠ることができました。環境が大きく変化したためか、引っ越してからずっと不眠症が続いているため、不眠症を完全に乗り越えるまで続けていくつもりです。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。