悪いところは特にないかなぁ。乗り換えでこっちの薬に変えたけど処方された薬との違いが分からない程度にはちゃんと効果を感じることができるのも最高でしたね。

左記クレジットカード、銀行振込、コンビニ決済に対応






更新日:2025/6/15
レクサプロ・ジェネリックは、うつ病治療のために病院で処方されることがあるレクサプロのジェネリック医薬品です。
初回向け低用量の5mg錠、維持量として服用できる10mg錠、20mg錠を選べるという特徴があります。
| メーカー | シプラ(Cipla) |
|---|---|
| 有効成分 | エスシタロプラムシュウ酸塩 |
| 効果 | 不安障害(GAD)・うつ病(MDD)の改善 |
| 副作用 | 吐き気や不眠など |
| 用法 | 1日1回、夕食後に服用 |
エスシタロプラムシュウ酸塩は、SSRI(選択的セロトニン再取り込み阻害薬)に分類される有効成分です。気持ちを楽にすることで抑うつ状態を解消するセロトニン(神経伝達物質)の濃度を高める作用があります。
レクサプロ・ジェネリックには、1錠あたりの配合量が異なる5mg錠、10mg錠、20mg錠の3種類があります。

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 35円 | 3,560円 | 106pt | |
| 200錠 | 31円 | 6,260円 | 187pt |
| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 49円 | 4,960円 | 148pt | |
| 200錠 | 40円 | 8,060円 | 241pt |
| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 69円 | 6,960円 | 208pt | |
| 200錠 | 59円 | 11,960円 | 358pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

レクサプロ・ジェネリック 5mg x 100錠
3,560円
ポイント:106pt
10,000円以上購入で送料無料
在庫あり

悪いところは特にないかなぁ。乗り換えでこっちの薬に変えたけど処方された薬との違いが分からない程度にはちゃんと効果を感じることができるのも最高でしたね。
ん~あんまり効いている感じはないかも・・・。
服用を続けることで、少しずつ気分の落ち込みや不安が和らぎ、前向きな気持ちが戻ってくることが多いです。レクサプロ・ジェネリックは脳のセロトニンに作用して、感情の安定を促す働きがあります。
レクサプロ・ジェネリックはパキシルと比較しても、同等の効果があることが確認されています。特に副作用が少ないという点で、より使いやすい選択肢とされることもあります。どちらを選ぶかは症状や体質により異なります。
軽度のうつ症状にもレクサプロ・ジェネリックは効果があります。10mgという低用量から始められるので、症状が軽くても無理なくスタートでき、必要に応じて増量することで対応可能です。
国内の臨床試験で、社会不安障害に対するレクサプロ・ジェネリックの有効性が確認されており、症状の改善率も時間経過とともに向上したことが示されています。特に52週間の長期試験で継続的な効果が見られました。
レクサプロ・ジェネリックは1日1回、夕食後に水で飲みます。通常は10mgから始め、必要に応じて20mgまで増量されることがあります。毎日同じ時間に服用を続けることが効果を安定させるポイントです。
うつ症状が酷い場合でも最初は10mgから始めるのが一般的です。様子を見てから、必要に応じて20mgまで増量していきます。そのため、最初から一気に多く服用するといったケースはまずありません。
レクサプロは錠剤として服用する設計になっており、粉砕すると薬の安定性や吸収に影響が出る可能性があります。服用が難しい場合は、医師または薬剤師に相談しましょう。
効果が出てきたからといって自己判断で薬を減らすのは避けるべきです。減薬や中止は、状態を見ながら医師と相談して徐々に減らしていくことが一般的です。
全ての人に使えるわけではありません。成分にアレルギーがある人、心臓病がある人、特定の薬(MAO阻害剤、ピモジド)を飲んでいる人には使えません。服用前に医師へ相談が必要です。
男性の場合、射精障害や性欲の低下といった副作用が出ることがあります。治療中に気になる変化があれば、無理に我慢せず医師に相談するようにしましょう。
急な中止でめまいや吐き気、不安感などの離脱症状が出ることがあります。そのため、症状が改善してきたらからといって自己判断で急に服用を中止したりするようなことはしないでください。
長期投与においても最も多い副作用は悪心、傾眠、頭痛などであり、重篤な副作用は少ないと報告されています。ただし体質によって違いがあるので、定期的な診察で確認が必要です。
| 1日の服用回数 | 1回 |
|---|---|
| 1回の服用量 | 10mg |
| 服用のタイミング | 夕食後 |
| 服用間隔 | 毎日 |
| 商品名 | パキシル・ジェネリック | ゾロフト | ベネジスXR | モノリスSR | アゴプレックス | アナフラニール | セントジョーンズワート | プリモックス |
|---|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |  |
| 特徴1 | ・症状に応じて4種類から選べる | 日本で実績があるジェイゾロフトの海外版 | ・神経伝達物質に影響を与えて気分をコントロールする | ・抗うつ薬のサポートにも使える | ・睡眠障害にも効果が期待できる | ・昔から国内で処方されている抗うつ剤 | ・軽度から中等度のうつ病の治療に有効 | ・実績が豊富で知名度が高い成分を配合 |
| 特徴2 | ・パキシルのジェネリックで安価 | 他の薬との併用で相互作用が起こりにくい | ・国内の抗うつ薬と同成分を配合している | ・不安定な気分を効果的に安定させる | ・従来品よりもさらに副作用の心配が少ない | ・軽度の副作用はあるものの高い効果を持つ | ・うつ病に伴う不眠症の改善にも期待できる | ・根本的な部分から不安や緊張を和らげる |
| 内容量 | 10mgx100錠 | 50mgx30錠 | 37.5mg28錠x1箱 | 300mg100錠x1箱 | 25mg50錠x1箱 | 10mg30錠x1箱 | 375mg120錠x1本 | 25mgx100錠 |
| 価格 | 9,460円 | 2,660円 | 3,560円 | 4,260円 | 6,560円 | 4,460円 | 4,260円 | 4,860円 |
| 5%以上 | 1〜5%未満 | 1%未満 | 頻度不明 | |
| 全身症状 | 倦怠感 | 異常感 | 無力症、浮腫、熱感、発熱、悪寒、疲労、体重増加、体重減少 | |
| 過敏症 | 発疹、湿疹、蕁麻疹、そう痒 | アナフィラキシー反応、血管浮腫 | ||
| 精神神経系 | 傾眠(22.6%)、浮動性めまい、頭痛 | あくび、不眠症、体位性めまい、感覚鈍麻、易刺激性(いらいら感、焦燥) | アカシジア、睡眠障害、異常夢(悪夢を含む)、激越、不安、錯乱状態、躁病、落ち着きのなさ、錯感覚(ピリピリ感等)、振戦、リビドー減退、歯ぎしり | パニック発作、精神運動不穏、失神、幻覚、神経過敏、離人症、ジスキネジー、運動障害、無オルガズム症 |
| 消化器 | 悪心(20.7%)、口渇 | 腹部不快感、下痢、食欲減退、腹痛、嘔吐、便秘 | 腹部膨満、胃炎、食欲亢進、消化不良 | |
| 循環器 | 動悸 | 起立性低血圧、QT延長 | 頻脈、徐脈 | |
| 血液 | 赤血球減少、ヘマトクリット減少、ヘモグロビン減少、白血球増加、血小板増加、血小板減少、鼻出血 | 出血傾向(斑状出血、消化管出血等) | ||
| 肝臓 | AST・ALT・Al-P・γ-GTP・ビリルビンの上昇等の肝機能検査値異常 | 肝炎 | ||
| 筋骨格系 | 関節痛、筋肉痛、肩こり、こわばり | |||
| 泌尿器・生殖器 | 排尿困難、尿蛋白陽性、射精障害 | 頻尿、尿閉、不正出血、勃起不全、射精遅延 | 持続勃起症、月経過多 | |
| その他 | 回転性めまい、耳鳴、多汗症 | 副鼻腔炎、味覚異常、脱毛、コレステロール上昇、血中ナトリウム低下、乳汁漏出、胸部不快感、寝汗、羞明、霧視、過換気、尿糖陽性 | 視覚異常、散瞳、高プロラクチン血症 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
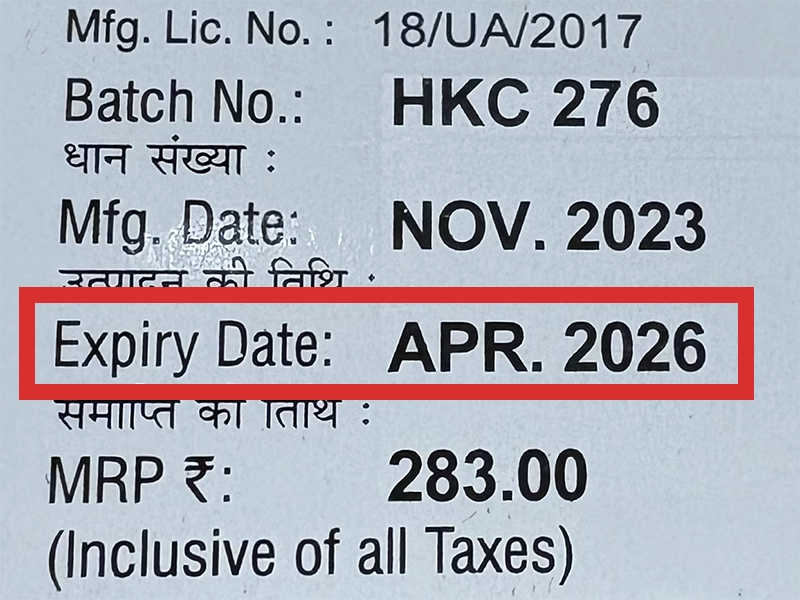
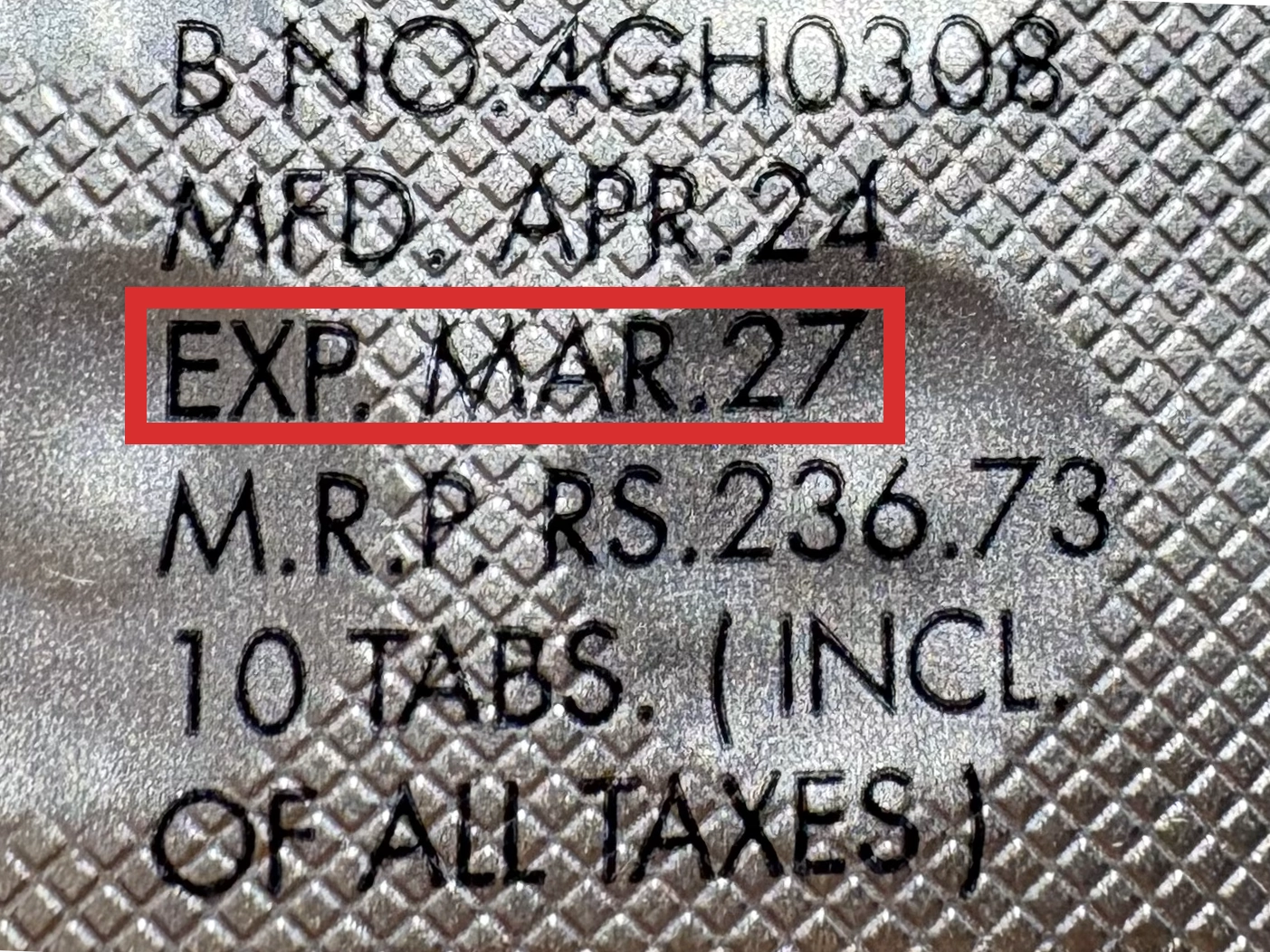
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
悪いところは特にないかなぁ。乗り換えでこっちの薬に変えたけど処方された薬との違いが分からない程度にはちゃんと効果を感じることができるのも最高でしたね。
今まで使ってきたものより断然いいと思う。レクサプロ自体は使ったことはないんだけども、ジェネリックでしっかり効果を感じることができているから今後も使おうかなと思ってる。
レクサプロ・ジェネリック、今回リピート購入なので前回の購入時のレポートだけさせてもらいます。1日1回の服用ですがしっかり効きます。服用量は自分は1錠ですが、増減できるのもありがたいです。前回購入が100錠で、3ヶ月ちょっと使った感じとしては効き目は強めで副作用は大きくは感じないという印象。使い勝手は悪くないので今回はこのままリピートさせてもらいました。
レクサプロのジェネリックということでポチってみましたっ!不安障害があるんで、レクサプロを飲んでいないと結構毎日が大変な感じになるんですけど、このジェネリックに変えてみてもレクサプロを飲んでるときと同じような感じなので非常に助かってます。外出も不安がある私にとって、自宅からでも購入できるのはかなり助かっています!今後も鬼リピしようと思ってます。
普段はレクサプロを使っていたんですけど、病院に行く手間がどうしても苦痛だったので、こちらで注文させてもらいました。最初は色々と不安もあったんですけど、レクサプロ・ジェネリックはレクサプロを使ってる時とほとんど変わらない感じで使えてますし、このまま継続して使わせてもらおうかと思ってます。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。