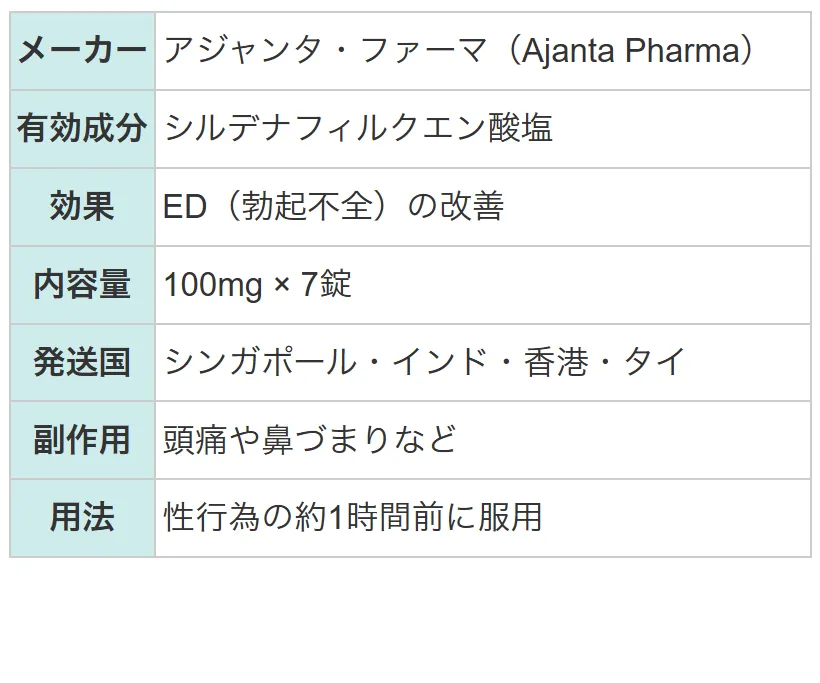
| 1日の服用回数 | 1回 |
|---|
| 1回の服用量 | 25~50mg |
|---|
| 服用のタイミング | 性行為の約1時間前 |
|---|
| 服用間隔 | 24時間 |
|---|
します。
初めてカマグラ発泡錠を服用する場合は、最少用量である1/4錠から服用を開始し、服用後の効果や副作用の有無を確認しながら、次回以降に1/2錠への増量を検討するようにしてください。
服用するタイミングは性行為の約1時間前の空腹時となっています。
水に溶かす際は、溶け残りがないように入念にかき混ぜてください。
また、1日の服用上限回数は1回となっているため、連続して服用したい場合でも最低24時間以上の間隔を空ける必要があります。
カマグラ発泡錠を服用する際の注意点
食事の影響を受けてしまう成分を配合しています。そのため、食事をしてからの服用だと十分な効果を得ることができなかったり、効果が出るまでの時間が長くなってしまうことがあります。
また、服用中の過度な飲酒は副作用のリスクを高めたり、酔いやすくなってしまうため
飲酒量を普段よりも少量で抑えたり、飲酒自体を控えるようにしましょう。
カマグラ発泡錠と服用方法が異なるシリーズ商品
カマグラ発泡錠には
カマグラや
カマグラPOLOなど、服用方法が異なるさまざまな種類があります。
これらは すべて同じ成分を配合しているため、同様の効果に期待できるようになっているので、自身の好みの服用方法に合わせて好きなものをお選びいただけるようになっています。
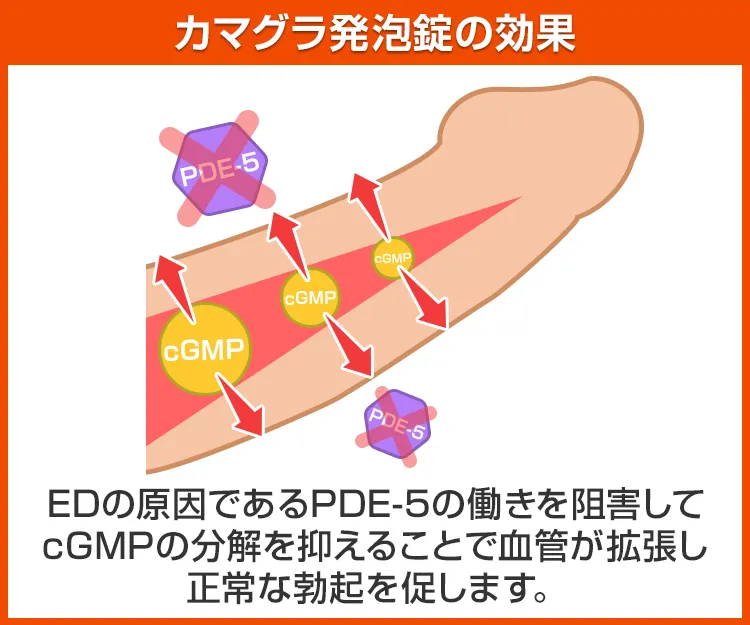
カマグラ発泡錠の効果
体内に存在する物質や酵素のうち、勃起と深い関係があるのがcGMP(環状グアノシン一リン酸)とPDE-5(ホスホジエステラーゼ5)です。
cGMPは性的な興奮などで増え、血管を広げて男性器を勃起へと導き、PDE-5はcGMPを分解することで勃起を鎮めます。
有効成分であるシルデナフィルは
PDE-5の働きを阻害することで、勃起を鎮める作用を抑えて正常な勃起やその維持を助けることで、EDを改善へと導きます。その他の効果も期待できるカマグラ発泡錠のシリーズ商品
カマグラシリーズの中には、EDだけでなく早漏に対しても効果を発揮する商品があります。
それが
スーパーカマグラです。
有効成分としてシルデナフィルだけでなく早漏に対して効果を発揮するダポキセチンを配合しており、EDと併発しやすい早漏にも有効な商品となっています。
| | 1%以上 | 0.1〜1%未満 | 0.1%未満 | 頻度不明 |
| 循環器 | 血管拡張(ほてり、潮紅)(5.78%) | 胸痛、動悸、頻脈 | 高血圧、不整脈、不完全右脚ブロック、末梢性浮腫 | 心筋梗塞、低血圧、失神 |
| 精神・神経系 | 頭痛(3.87%) | めまい、傾眠、昏迷 | 異常感覚、下肢痙攣、記憶力低下、興奮、緊張亢進、錯乱、思考異常、神経炎、神経過敏、神経症、不安、不眠症、無気力 | |
| 肝臓 | | AST増加 | ALT増加、LAP上昇、LDH増加、血中トリグリセリド増加、γ-GTP増加、血清リン脂質上昇、血中アミラーゼ増加、血中アルブミン減少、血中ビリルビン増加、総蛋白減少 | |
| 消化器 | | 悪心、胃腸障害、口渇、消化不良、腹痛 | おくび、胃炎、胃不快感、下痢、口唇乾燥、舌障害、白舌、腹部膨満、便秘、嘔吐、嚥下障害 | |
| 泌尿・生殖器 | | | 陰茎痛、射精障害、朝立ちの延長、半勃起持続 | 勃起の延長、持続勃起、尿路感染、前立腺疾患 |
| 呼吸器 | | 鼻炎 | 呼吸障害、鼻閉、咽頭炎、喘息 | 鼻出血、気道感染症、副鼻腔炎 |
| 筋・骨格系 | | 関節痛、筋肉痛 | 骨痛、背部痛 | |
| 皮膚 | | 発疹 | そう痒症、眼瞼そう痒症、脱毛症、男性型多毛症、発汗、皮膚乾燥、皮膚障害、紅斑 | |
| 血液 | | | ヘマトクリット減少、ヘマトクリット増加、ヘモグロビン減少、リンパ球減少症、リンパ球増加症、好酸球増加症、赤血球減少症、赤血球増加症、白血球増加症 | |
| 感覚器 | | 眼充血、結膜炎、彩視症、視覚障害 | 眼乾燥、眼痛、屈折障害、光視症、味覚異常、味覚消失、流涙異常、羞明 | 霧視、視力低下、網膜出血、網膜静脈閉塞、突発性難聴 |
| その他 | | CK増加、疼痛、熱感 | BUN増加、インフルエンザ症候群、リンパ節症、血中ナトリウム減少、血中リン増加、体重増加、血中尿酸増加、ウロビリノーゲン陽性、尿中ブドウ糖陽性、尿中赤血球陽性、尿中蛋白陽性、疲労、無力症 | 過敏性反応、感染症 |
動悸、頻脈、視覚異常などの症状があらわれることがあります。
服用によって、急激な血圧変化がみられる可能性があるので十分注意してください。
そのほかに副作用と思われる症状があらわれた場合は、速やかに服用を中止してください。
服用方法や副作用・併用禁忌・注意のご説明には、商品の説明書の他に、次のサイトを参考にしています。
医療用医薬品 : バイアグラ
パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。










































商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。