健康診断で尿酸値を測る様になったのが30歳からでその時から既に8以上ありそれから10数年!ついに9を越え薬を飲まなくてはいけなくなりました。毎日忙しく病院になかなか行けないので困り果てたところこのサイトを発見し購入!飲み始めて2ヶ月くらいして健康診断があったのですがなんと!5.8でした!人生初の数値にビックリです。お試しだと思い1セットしか買わなかったのですが次はまとめ買いしようと思います。

左記クレジットカード、銀行振込、コンビニ決済に対応




更新日:2025/4/5

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 50錠 | 125円 | 6,260円 | 187pt | 売り切れ |
| 100錠 | 79円 | 7,960円 | 238pt | 売り切れ |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

ザイロリック・ジェネリック 300mg x 100錠
7,960円
ポイント:238pt
10,000円以上購入で送料無料
売り切れ

健康診断で尿酸値を測る様になったのが30歳からでその時から既に8以上ありそれから10数年!ついに9を越え薬を飲まなくてはいけなくなりました。毎日忙しく病院になかなか行けないので困り果てたところこのサイトを発見し購入!飲み始めて2ヶ月くらいして健康診断があったのですがなんと!5.8でした!人生初の数値にビックリです。お試しだと思い1セットしか買わなかったのですが次はまとめ買いしようと思います。
一回目は100mg84錠を買いました。でも飲んでたらすぐになくなったので、二回目には300mgの100錠を買いました。300mgにしてから間もなく体全体が不調になり、細かくいうと貧血みたいな状態がよく続くようになっていきました。おそらくですが、分量が変わったせいだと思います。貧血は仕事へもろに影響が出てしまうので、近々薬を替える他ないかなと考えてます。
体の中で尿酸がたまりすぎないようにして、痛風発作が起こるのを予防するために使われます。発作を減らすためには、長く続けることが大切です。
ザイロリックは、飲み始めてから数日から1週間くらいで血中尿酸値が下がり始めると言われています。ただし、安定した効果を得るにはしばらく時間がかかることもあります。
長期間飲み続けても効果が落ちにくい薬です。何年も安定して尿酸値をコントロールできることが確認されています。
痛風発作の最中にザイロリック・ジェネリックを飲み始めると、かえって症状を悪化させることがあるので注意が必要です。通常は発作が落ち着いてから服用を始めるのが基本となるので、発作を抑えるために使うのは避けてください。
服用量を自己判断で増やすのは絶対にやめましょう。尿酸値の下がり方を見ながら、必要に応じて先生が調整してくれるので、勝手に変更しないことが大切です。
尿酸値の数値がよくなっても自己判断でやめてはいけません。尿酸値を安定させ続けるために、先生に指示された期間はきちんと飲み続ける必要があります。
飲み始めた直後は、一時的に痛風発作が起こることがあります。もし痛みが出たらすぐに医師に相談するようにしてください。無理にやめる必要はない場合もあります。
はい、服用している間は、尿をしっかり出して尿酸を排泄するために、水分をたくさんとることが推奨されています。
血液をサラサラにする薬(ワルファリンなど)や特定の抗がん剤と一緒に飲むと注意が必要です。ほかに飲んでいる薬があれば必ず先生に伝えてください。
発熱した場合は、重いアレルギー反応の可能性もあるので、できる限り速やかに医療機関に相談してください。決して自己判断で様子を見たりすることはないようにしてください。
はい、服用している時は尿をたくさん出すために水分をしっかりとることが大切です。これによって尿路結石などのリスクを減らすことができます。
飲み始めてもすぐに効果が出ないことがあります。ですが、適切に継続していくことで徐々に尿酸値が下がっていきます。焦らず先生の指示通りに飲み続けましょう。
| 1日の服用回数 | 2~3回 |
|---|---|
| 1回の服用量 | 200~300mg |
| 服用のタイミング | 食後 |
| 服用期間 | 6時間以上 |
| 商品名 | ザイロリック | コルヒチン | ポトレート | フェブトップ | ズリック40 | コルヒチンリア |
|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |
| 特徴1 | ・標準薬として使用されている尿酸降下薬 | ・高尿酸血症に伴う痛風症状の緩和に有効 | ・尿や体液をアルカリ化 | ・症状がない無症候性高尿酸血症にも有効 | ・尿酸の生成を抑えて痛風を予防 | ・白血球の活動を制限 |
| 特徴2 | ・有名な製薬会社のGSKファーマが販売 | ・痛風症状の予防を目的として使用することも可能 | ・尿路結石の予防にも有効 | ・ザイロリックよりも強力 | ・アロプリノールより強力 | ・炎症反応を抑制 |
| 内容量 | 100mgx100錠 | 500mcg30錠x1本 | 540mg100錠x1箱 | 40mg30錠x1箱 | 40mg30錠x1本 | 0.5mg30錠x1箱 |
| 価格 | 5,360円 | 4,460円 | 3,160円 | 4,360円 | 3,160円 | 4,260円 |
| 0.1〜5%未満 | 0.1%未満 | 頻度不明 | |
| 過敏症 | 発疹 | そう痒、関節痛 | |
| 血液 | 貧血 | 白血球減少、紫斑、好酸球増多、リンパ節症 | |
| 腎臓 | 腎機能異常 | ||
| 消化器 | 食欲不振、胃部不快感、軟便、下痢 | 口内炎 | |
| 全身症状 | 全身倦怠感 | 浮腫 | 脱力感 |
| その他 | 脱毛 | CK上昇、味覚障害、女性化乳房、末梢神経障害 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
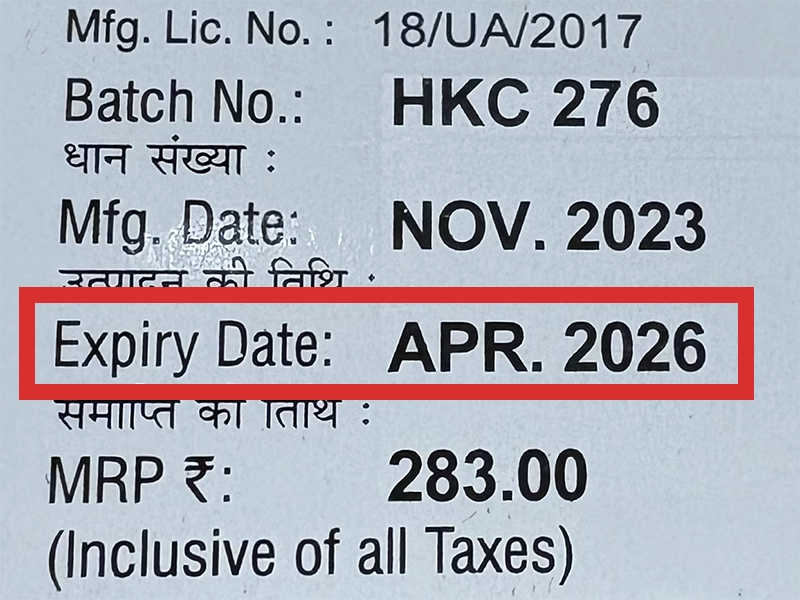
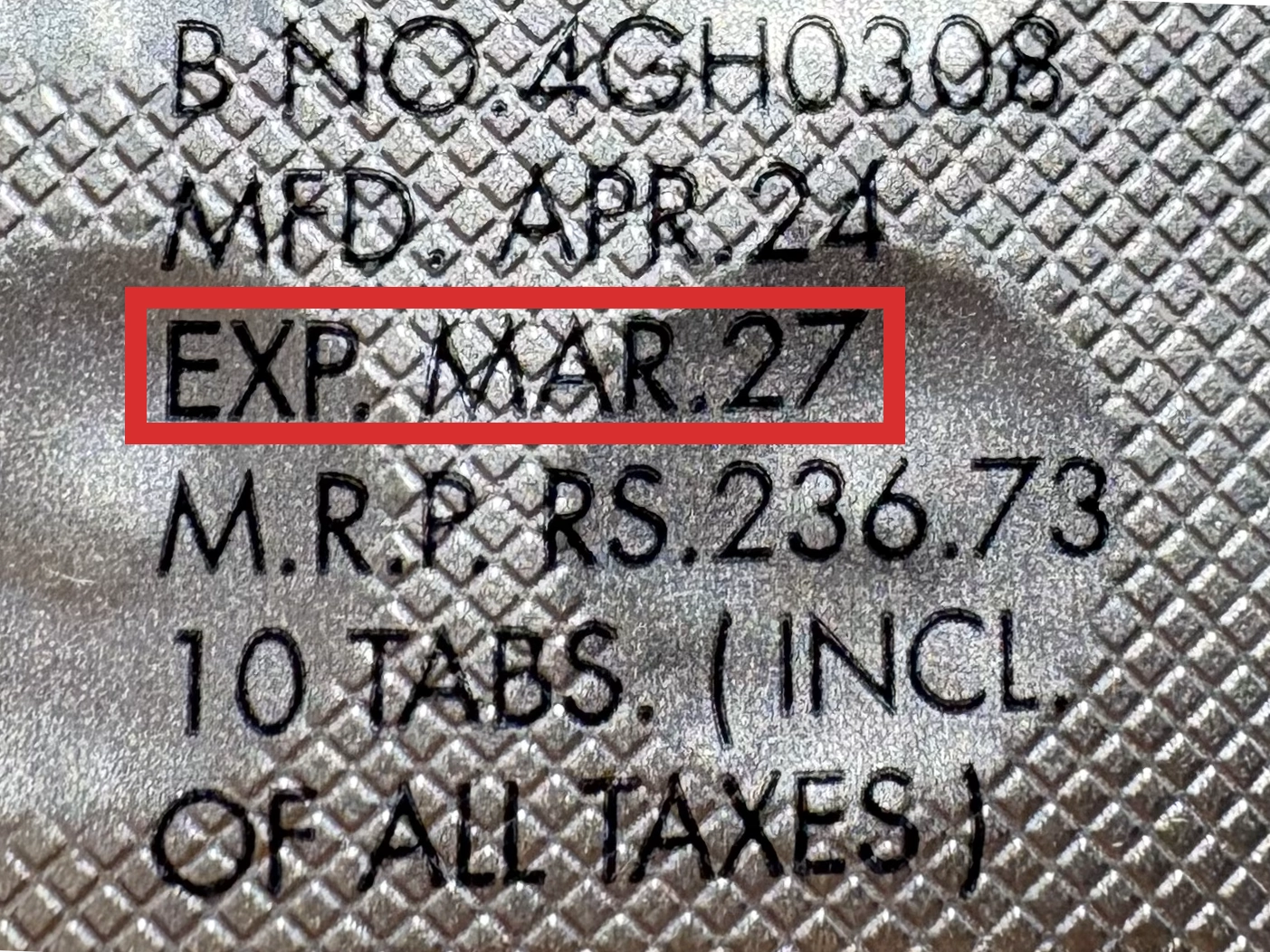
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
健康診断で尿酸値を測る様になったのが30歳からでその時から既に8以上ありそれから10数年!ついに9を越え薬を飲まなくてはいけなくなりました。毎日忙しく病院になかなか行けないので困り果てたところこのサイトを発見し購入!飲み始めて2ヶ月くらいして健康診断があったのですがなんと!5.8でした!人生初の数値にビックリです。お試しだと思い1セットしか買わなかったのですが次はまとめ買いしようと思います。
ザイロリック・ジェネリックを服用するようになって約2ヶ月、まだこれといった効果は感じてませんが、昨日尿酸値の結果を見てみると若干下がっていました。このままいくと来月くらいには正常値になっていることでしょう。検査が楽しみでもあります。
ジェネリックに対して明確な理由はないのですが抵抗がありました。処方されていたザイロリックを服用していたのですが家の事情でなかなか病院に行けず薬が切れ痛みに耐える日々でした。ザイロリックのジェネリックならこちらで買えると知り注文しました。服用し痛みが引いた時は涙が出ました。ジェネリックや個人輸入の偏見は捨てるべきだと思いました。
寒さのせいで関節痛が酷いのかと思ったのですが、病院に行った結果…痛風でした。通院して処方箋をもらい薬を買っていたのですが、一家の大黒柱としてそんなに暇じゃないです。なんとかならんかとネットで調べたらこちらのサイトでザイロリックのジェネリックが購入できるじゃないですか、非常に助かりました。
長年痛風と戦っておりましたが、ここ数年はザイロリックに助けられていました。最近になって個人輸入の存在を知ったのでジェネリックに切り替えたのは先日ですが、問題なさそうです。これは本当によく効いてくれるので痛風持ちの方はぜひお試しあれ
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。