浮き沈みが激しかった気持ちが改善され、とても生活がしやすいです。自分には副作用がないので飲みやすく、通院もしなくていいので便利ですね。今後もお世話になります。

左記クレジットカード、銀行振込、コンビニ決済に対応


更新日:2025/6/20
| 商品名 | (Emre Ecza)マイソリン | ゾニセップ | フィコンパ | テグレトール・ジェネリック | トピロール | プリミドン | テグレトールCR |
|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |
| 特徴1 | ・塩化物イオンの流入を促進 | ・大容量かつ低価格で長期服用に最適 | ・神経の過剰な興奮を抑制 | ・国内で処方されている医薬品の後発品で安心 | ・幅広い作用で抗てんかん効果を発揮する | ・塩化物イオンの流入を促す | ・てんかんの発作を予防 |
| 特徴2 | ・神経の過剰な興奮を抑制 | ・てんかんやパーキンソン病にも効果的 | ・グルタミン酸受容体の活性を阻害 | ・てんかんや躁うつ病などにも有効 | ・世界約100カ国で使用されている抗てんかん薬 | ・抗けいれん作用、催眠・鎮静作用などをあらわす | ・神経の異常な興奮を抑制 |
| 内容量 | 250mg30錠x1箱 | 25mgx50錠 | 2mg28錠x1箱 | 200mgx25錠 | 25mgx100錠 | 250mg100錠x1本 | 200mg60錠x1箱 |
| 価格 | 4,660円 | 2,960円 | 5,160円 | 4,160円 | 4,960円 | 9,260円 | 6,460円 |

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 100錠 | 191円 | 19,160円 | 574pt | 売り切れ |
| 200錠 | 184円 | 36,860円 | 1,105pt | 売り切れ |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

ラミトール 200mg x 200錠
36,860円
ポイント:1,105pt
10,000円以上購入で送料無料
売り切れ

浮き沈みが激しかった気持ちが改善され、とても生活がしやすいです。自分には副作用がないので飲みやすく、通院もしなくていいので便利ですね。今後もお世話になります。
効き目があるように思えないので服用量を増やしてみると、副作用が強くてつらいです…慣れるかもしれないと思ったのですが、無理そうなので他の薬に切り替えようと思います。
てんかん薬の中には、副作用として倦怠感などがあらわれるものがあります。そのため、服用する薬によっては倦怠感を感じる可能性があります。あまりにも症状が酷いといった場合には、服用する薬の変更の相談などを行うようにしましょう。
てんかん薬の種類の中には、毛が薄くなるといったものはあります。逆に濃くなる種類の治療薬っていうものはないと考えられます。そのため、毛が濃くなったという場合には、てんかん薬以外の何か白が原因と考えられます。
てんかん薬を多量に服用したりした場合には、記憶力に影響を及ぼす可能性があったりします。ですが、適切にてんかん薬を使った場合にはそのリスクはほとんどないため、適切な用法用量を守って服用するようにしてください。
てんかんの場合に運転免許を取得するには、薬の服用は関係ありません。運転に支障があるような発作が数年にわたってない場合に取得できるとされているため、適切な治療を行い長期に渡って発作などが起きていない場合には取得することは可能といえます。
妊娠すること自体は可能ですが、催奇性の高い薬の場合はその分だけ奇形などのリスクは高まってしまいます。そのため、妊娠を希望する場合以外は適切な避妊を行う必要があります。また妊娠を希望する場合には、事前に医師に相談して妊娠とてんかん治療を両立することができる薬への変更などを行うことが重要になります。
治療薬の中止や再開を自己判断で行うのは大きなリスクがあります。医師と相談した上で適切な用法用量を守ってご使用ください。そのため、再開する場合であっても自己判断で行うのではなくまずは相談するようにしてください。
| 1日の服用回数 | 1回 |
|---|---|
| 1回の服用量 | 25~50mg |
| 服用のタイミング | 指定なし |
| 服用間隔 | 24時間以上 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
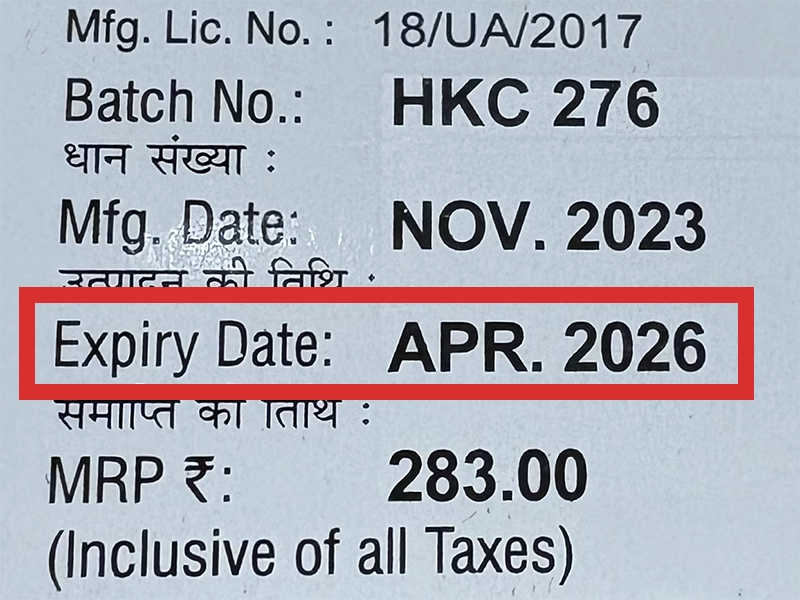
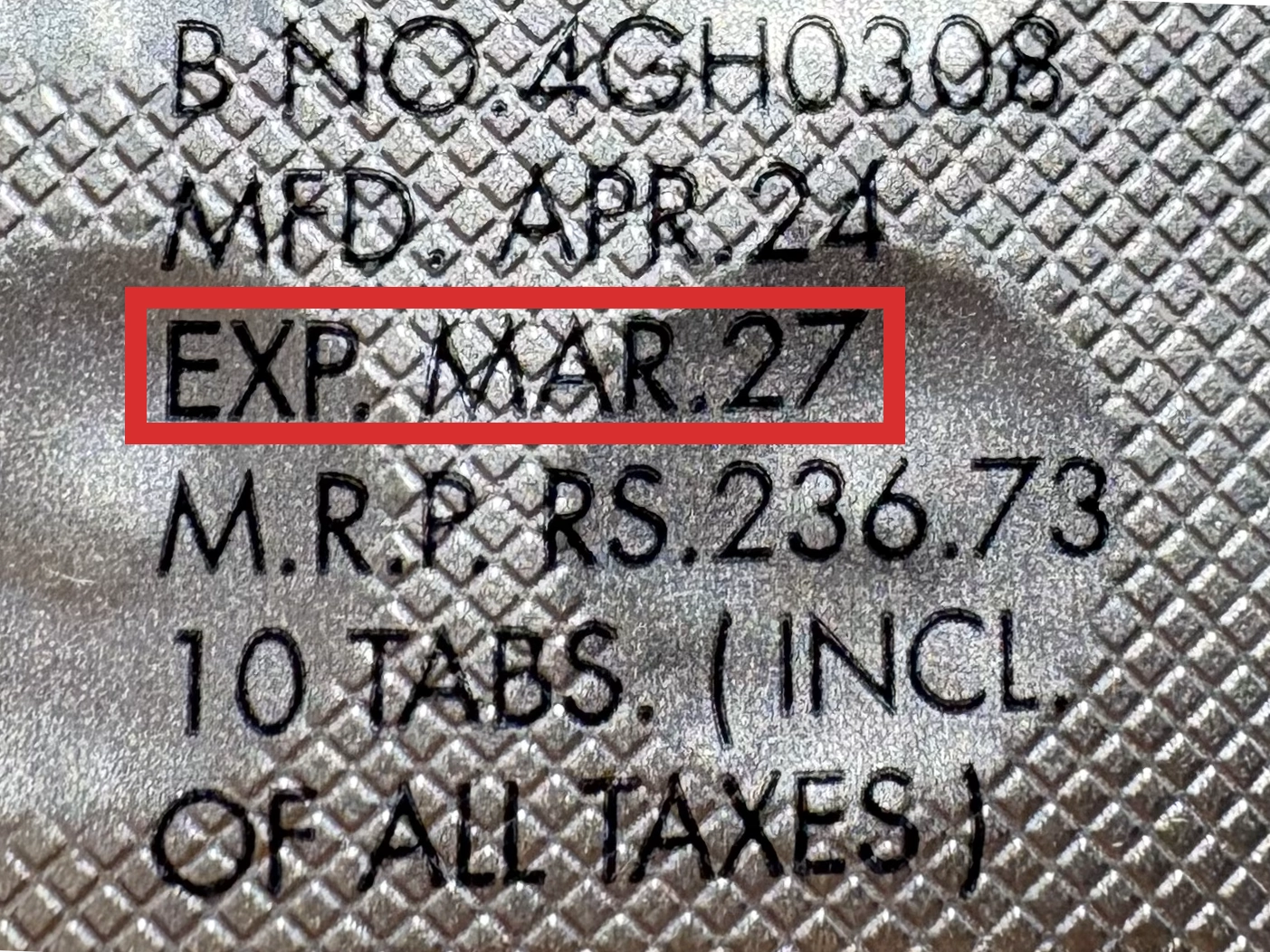
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
浮き沈みが激しかった気持ちが改善され、とても生活がしやすいです。自分には副作用がないので飲みやすく、通院もしなくていいので便利ですね。今後もお世話になります。
効き目があるように思えないので服用量を増やしてみると、副作用が強くてつらいです…慣れるかもしれないと思ったのですが、無理そうなので他の薬に切り替えようと思います。
激しい感情を抑え、気持ちを落ち着かせてくれます。効き目はいいですが、その分副作用も軽くありました。飲み慣れてしまえばなんてことないです。
こういう薬がネットで買えるって凄いですね。通院するのが辛い私には助かります。梱包も丁寧にしてあるので安心です。
てんかんの発作を繰り返してたけどラミクタール飲んでからは発作の回数がかなり減った!通販で買えるのは楽だし本当に便利で助かってます!
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。