定期的に休薬期間を挟みつつ、トレーニングに活用しています。手軽にトレーニングの効果を高め、全体的な筋肉量を増やすことができるので便利だなと感じております。副作用が少ないわりに効果を実感しやすいというのも評価できます。

左記クレジットカード、銀行振込、コンビニ決済に対応



更新日:2025/6/10
スタノゾロールは、今よりもっと強い体作りをサポートする医薬品です。
有効成分には、筋肉増強作用だけでなく、骨密度を高めたり造血機能をアップさせたりする作用があります。
強い筋肉、強い骨を作り、血流を増加させて根本からパワフルに生まれ変わることができます。
| メーカー | エリス・オークネット・ヘルスケア(Eris Oaknet Healthcare) |
|---|---|
| 有効成分 | スタノゾロール |
| 効果 | 筋肉増強 骨粗しょう症の改善(併用療法) |
| 副作用 | 頭痛や吐き気など |
| 用法 | 毎日一定の時刻に1回分を服用(長期投与不可) |
有効成分のスタノゾロールは、体内に取り込まれると男性ホルモンとして働く性質がある有効成分で、タンパク質同化性(アンドロゲン性)ステロイドに分類に分類されます。男性ホルモン増量による筋肉増強のほか、食事療法やカルシウムコントロールなどと組み合わせることにより、骨粗しょう症の方の骨量増加にも期待できます。
スタノゾロールは、1錠あたり2mg配合されています。

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 20錠 | 138円 | 2,760円 | 82pt | |
| 40錠 | 101円 | 4,060円 | 121pt | |
| 60錠 | 92円 | 5,560円 | 166pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

スタノゾロール 2mg x 20錠
2,760円
ポイント:82pt
10,000円以上購入で送料無料
在庫あり

定期的に休薬期間を挟みつつ、トレーニングに活用しています。手軽にトレーニングの効果を高め、全体的な筋肉量を増やすことができるので便利だなと感じております。副作用が少ないわりに効果を実感しやすいというのも評価できます。
効率よく筋肉をつけれるかと思ったが、毛が昔よりも抜けるようになってきた。楽して筋肉がつけれたとしてもハゲになるのは嫌だわ。
スタノゾロールはアナボリックステロイドの一種で、タンパク質合成を促進することで筋肉量を増やす作用があるとされています。本来は医療目的で使われていた成分ですが、筋力アップや体つきを変えたいという理由で不適切に使用されるケースも多く、効果と同時に副作用リスクの理解も必要です。
筋肉は基本的に負荷をかけて成長するものなので、スタノゾロールの作用だけで大きな変化が起きることはありません。補助的な合成作用は期待されますが、運動や栄養管理と組み合わせなければ十分な結果は得られないと考えられています。
スタノゾロールはホルモンに直接作用する薬剤で、一般的な筋トレサプリとは全く異なります。摂取によって内分泌系に影響が出るため、効果は高くても副作用や健康リスクが大きい点が決定的な違いです。
短期間でも効果が出ることは報告されていますが、スタノゾロールはWADAの禁止物質であり、医療目的以外での使用は違法やリスクの高い行為とされています。肝機能障害やホルモンバランスの乱れといった副作用も短期間で出る可能性があるため、安易な使用は強く注意が必要です。
スタノゾロールは筋トレ前に飲んでも即効性があるわけではないため、基本的には決まった時間に毎日服用することが重要とされています。運動のタイミングに合わせて飲むというよりも、血中濃度を一定に保つための継続服用が前提です。
多めに服用しても筋力の増強効果が高まることはありません。適切な用法用量を守って使用するようにしてください。自己判断での適当な服用は副作用のリスクを高めるので、かならず適切な用法用量を守ってください。
基本的に食後が推奨されています。食後の服用で成分の吸収が安定するとされているためです。そのため、毎日の食事を摂った後に忘れないように服用するようにしましょう。
飲み忘れがあったからといっても、即日効果が完全に消失するといったことはありません。ですが、やはり飲み忘れないようにすることは何よりも大切なので、飲み忘れが起きないように注意しましょう。
スタノゾロールは経口摂取で肝臓を通過するため、他の薬剤と比べても肝機能への負担が大きいとされています。黄疸や肝障害、まれには肝腫瘍のリスクも報告されているため、肝機能に不安がある方や、長期間の使用を検討している場合は特に注意が必要です。
スタノゾロールはWADA(世界アンチ・ドーピング機構)の禁止薬物リストに常時掲載されており、競技中でなくても検出されればドーピング違反となります。使用時期に関係なく尿検査などで検出される可能性があるため、アスリートが使うことは絶対に避けるべきとされています。
一部の使用者から、気分が高揚しやすくなったり、怒りっぽくなったりするといった精神的な変化が報告されています。これはスタノゾロールが脳内のホルモンバランスに影響を与えるためとされており、特に大量または長期使用で顕著になるケースがあるようです。
肝機能障害や血栓症、心血管系への悪影響など、命に関わる可能性のある副作用が報告されています。とくに肝腫瘍や突然の血圧上昇といった変化は放置すると重篤化するリスクがあり、自己判断での使用は大きな危険を伴います。
| 1日の服用回数 | 1~3回 |
|---|---|
| 1回の服用量 | 2mg |
| 服用のタイミング | 食後 |
| 服用間隔 | 6時間以上 |
| 商品名 | アナドリン | メダナボル | オキシポロン | ウィンゾロン | ハロフルオックス | プリモノロン | アンドロフォルテクリーム | テストステロンジェル |
|---|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |  |
| 特徴1 | ・骨髄の機能を高める効果にも期待できる | 効率よく筋肉増強をサポートする | ・骨髄の機能を高める効果にも期待できる | 実績のある筋肉増強剤 | ・体脂肪を減らす効果にも期待できる | ・女性化作用や肝臓への負担が少ない | ・手軽に男性ホルモンを補給できる | 男性ホルモン補充で筋肉増強 |
| 特徴2 | ・カルシウムの排出量を抑えて骨を強化する | ボトル1本にたっぷり60錠入り | ・トレーニングのサポートにも最適 | 大容量(150錠)入りボトル | ・服用を中止しても一定期間、効果が持続する | ・従来の筋肉増強剤より安全性が高い | ・さっと使用することができる外用薬タイプ | 皮膚から直接有効成分を取り込めるクリーム |
| 内容量 | 10mgx60錠 | 10mgx60錠 | 50mgx60錠 | 10mgx150錠 | 5mgx120錠 | 25mgx150錠 | 5%x1箱 | 1% 5gx1本 |
| 価格 | 6,260円 | 4,360円 | 5,860円 | 6,060円 | 14,060円 | 29,160円 | 24,460円 | 1,800円 |
| 頻度不明 | |
| 全身症状 | 発熱、突然死、倦怠感、口渇、浮腫 |
| 肝・胆道系 | 胆汁うっ滞、黄疸、肝機能障害、肝不全、肝硬変、肝腫大、肝炎 |
| 感染症 | 乳腺炎、結膜炎、肺炎、敗血症、喉頭炎 |
| 筋骨格系 | 腱断裂、打撲、関節炎、関節痛、筋痛、筋けいれん、デュピュイトラン拘縮 |
| 血液・検査値異常 | 血中アルカリホスファターゼ上昇、出血時間延長、凝固時間延長、プロトロンビン値低下、白血球増加、ALT上昇、肝酵素上昇、体重増加、GFR低下 |
| 代謝・内分泌系 | 代謝性アルカローシス、食欲減退、食欲亢進、電解質異常、体液貯留、糖尿病、低血糖、高コレステロール血症、痛風 |
| 腫瘍 | 肝腺腫、肝細胞癌、前立腺癌 |
| 神経・精神系 | 脳梗塞、脳血管障害、一過性脳虚血発作、頭痛、頭蓋内圧亢進、舞踏病様運動、ジストニア、片麻痺、振戦、失語、無気力、傾眠、めまい、知覚異常、上位運動ニューロン障害、手根管症候群、発作、てんかん重積状態、攪乱、不安、せん妄、うつ、離人症、現実感喪失、幻覚、感情不安定、陶酔感、気分変動、性欲亢進、不眠 |
| 泌尿器・生殖器系 | 急性腎障害、腎不全、高窒素血症、腎尿細管壊死、乳腺線維嚢胞症、乳頭異常、乳房緊満感、乳房痛、月経異常、前立腺疾患、会陰痛、勃起不全、男性不妊、無精子症 |
| 呼吸器系 | 喘息、気管支けいれん、肺塞栓症、呼吸困難、喀血、肺出血、発声障害、鼻出血 |
| 皮膚・皮下組織 | 血管性浮腫、蕁麻疹、皮膚肥厚、多形紅斑、皮膚炎、湿疹、紅斑、角質除去、光線過敏症反応、掻痒、発疹、斑状丘疹性発疹、ニキビ、脱毛症、多汗症、多毛症、爪障害、皮下出血 |
| 循環器系 | 循環不全、血栓症、血栓性静脈炎、血管痛、フラッシング、出血、高血圧、巨細胞性動脈炎、静脈炎、血管炎 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
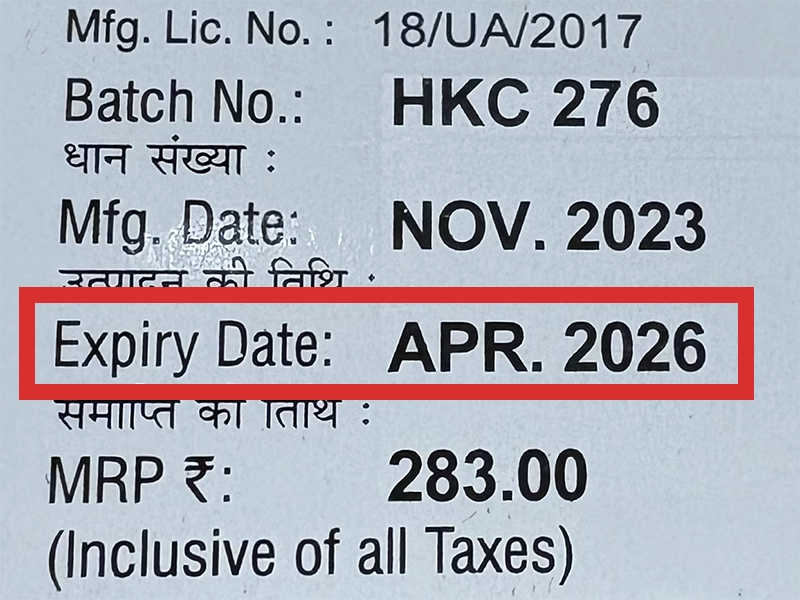
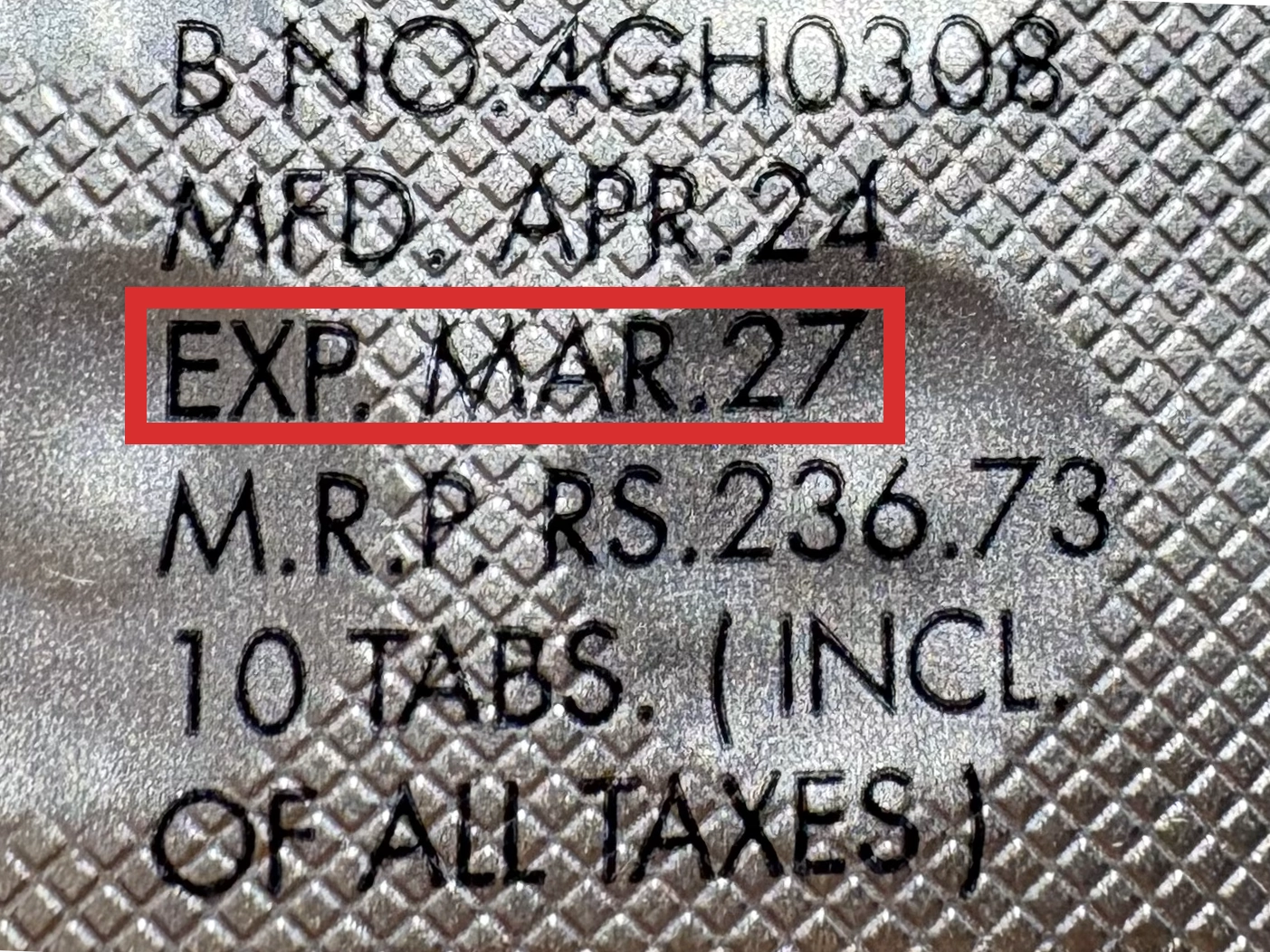
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
定期的に休薬期間を挟みつつ、トレーニングに活用しています。手軽にトレーニングの効果を高め、全体的な筋肉量を増やすことができるので便利だなと感じております。副作用が少ないわりに効果を実感しやすいというのも評価できます。
使ったあと、頭痛がちらほら起きたが、これのおかげで上手く筋肉を残しつつ脂肪を減らして身体を引き締めることができた。この手の薬を使うことには不安もあったが、思っていたような副作用は起きず、トレーニングに集中することができた。理想の身体にはまだ遠いため、引き続きこれを使いながらトレーニングに励む予定でいる。
結構しっかり黄色の錠剤だから、本当に飲んでも大丈夫なのか不安はありますが、かなり効率よく筋肉がつき、脂肪も落ちやすいです。ですが、飲むと倦怠感が出てくるため、トレーニングをするのが少し大変です。副作用がなかったらもっと使いやすいんだけどな・・・。
昔に比べるとニキビができやすくなったが、筋肉は格段につきやすくなった。筋疲労の回復にも最適だから、筋疲労対策目的で飲むのもありじゃないか?肌荒れが気になる人は使いづらく感じるかもしれないが、確実に筋肉がつくからオススメできる。
1日3回、1錠というペースで服用中です。3週間経過で、大体4kgほど増量しました!トレーニングの内容を見直さなくても、これまでどおりでしっかり筋肉がついてくれるので重宝しています。もう少し筋肉が欲しいと考えているので、このまま上手く付き合っていき、理想の筋肉のつき具合になるまで頑張る予定です。良いものと出会えました。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。