かなり歳も取ってきて更年期障害の症状が出始めていたので2年前くらいから飲み始めました。最初は指定の量を服用してたのですが私には少し薬の効果が強すぎるせいか副作用がかなり現れたので量を減らして服用していました。それからというもの服用を始める前は息切れや肩こり関節痛がひどかったのですが元気を取り戻し運動もこまめにこなせるようにまで回復しました!

左記クレジットカード、銀行振込、コンビニ決済に対応


更新日:2025/4/28

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 50錠 | 52円 | 2,600円 | 78pt | |
| 100錠 | 50円 | 5,000円 | 150pt | |
| 150錠 | 48円 | 7,200円 | 216pt |
| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 50錠 | 76円 | 3,800円 | 114pt | 売り切れ |
| 100錠 | 68円 | 6,840円 | 205pt | 売り切れ |
| 150錠 | 60円 | 9,120円 | 273pt | 売り切れ |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

グランダキシン・ジェネリック 50mg x 50錠
2,600円
ポイント:78pt
10,000円以上購入で送料無料
在庫あり

かなり歳も取ってきて更年期障害の症状が出始めていたので2年前くらいから飲み始めました。最初は指定の量を服用してたのですが私には少し薬の効果が強すぎるせいか副作用がかなり現れたので量を減らして服用していました。それからというもの服用を始める前は息切れや肩こり関節痛がひどかったのですが元気を取り戻し運動もこまめにこなせるようにまで回復しました!
しばらく飲んでみました。いつのまにか効いて、いつのまにか効果が切れているような薬でした。まだ重度のうつ病ではないので、強すぎる薬は飲みたくないんですけど、この薬はどの程度体に効いているのかがわからなくてやきもきします。副作用として眠気が何回も現れているので、全然効き目がないわけじゃないというのはわかりますが…。もっとふさわしい薬があったらそっちにしたいです。
自律神経失調症の治療で用いられる治療薬は対症療法に使用されます。そのため、自律神経失調症の症状を改善するといった効果は期待できます。ですが、自律神経失調症そのものの治療に対しての効果はないため、完全に治すといったことは難しいと言えます。
自律神経失調症の治療薬を服用している場合は、その間の妊娠は避けた方がよいとされています。そのため、薬の服用をしなくてもよくなった場合には妊娠しても問題はないと言えます。そうでない場合に妊娠を希望する時は、医師に相談するなどして胎児などに影響を及ぼしにくい治療薬に変更してもらうといった必要があります。
服用する薬の種類などによってや、自律神経失調症に悩む人によって改善までの期間が違います。数週間で症状が緩和していき、半年から1年でほぼ気にならないほどにまでなるという方もいれば、何年も服薬をしているけど薬をやめると症状が出るといった方までさまざまなので、一概にどの程度というのはいえません。
自律神経失調症の治療薬は病院での処方や個人輸入でのみ手に入れることができます。そのため、ドラッグストアなどでは取り扱っていないため、購入することもできません。
行くこと自体は問題ないといえますが、薬や自律神経失調症の影響によって、具合が悪くなってしまうといったリスクも踏まえて家族などと一緒に行くようにするとよいといえます。一人の場合や友人の場合、周りに迷惑をかけてしまう可能性もあるため、注意が必要です。
| 1日の服用回数 | 3回 |
|---|---|
| 1日の服用量 | 50mg |
| 服用のタイミング | 朝、昼、晩 |
| 服用間隔 | 6時間 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
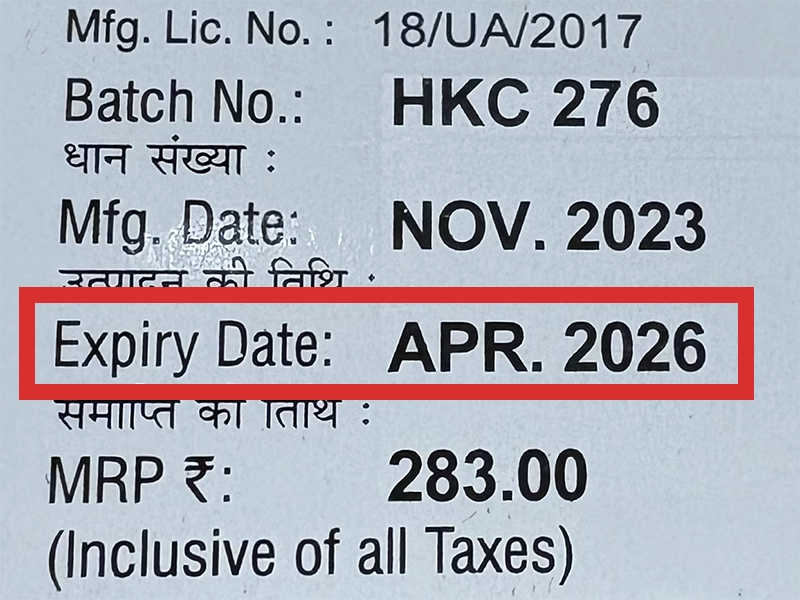
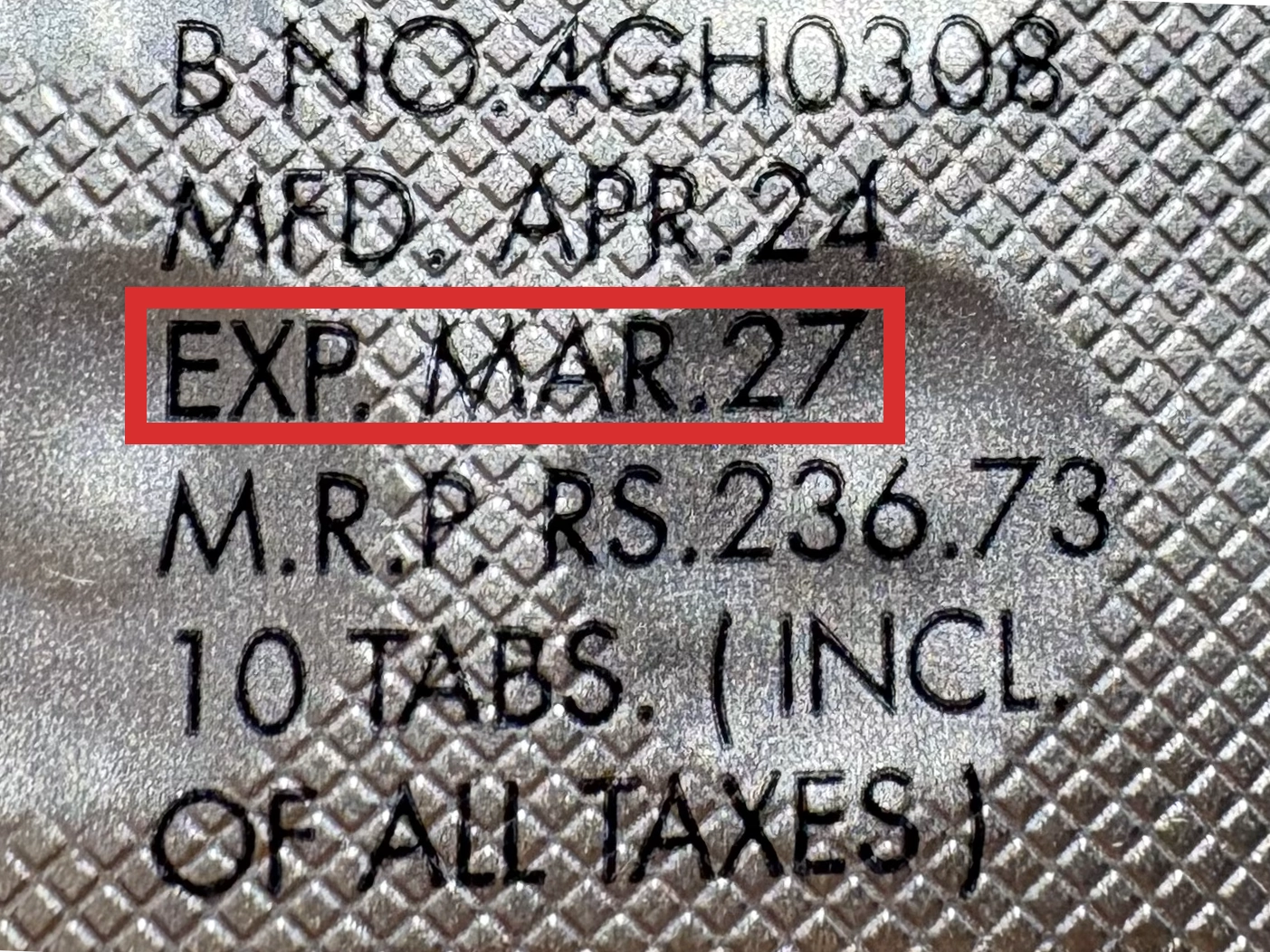
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
不安やイライラは減ったが、常に眠気に襲われる あと半分以上残ってるのはもったいないが 飲みながら他の薬を探すことにする
大きな変化は感じてませんが、感情の波が穏やかになったかもしれません。副作用らしき症状もとくに現れていませんので購入分(150錠)は飲みきってみます。
服用を開始してから約1年になります。40代後半から更年期症状が出てきましたが、今は心身ともにかなり調子がよく、友人からも「変わったね」とか「元気になったね」といわれるようになりました。最近は1日3回のところ1日2回にして服用してますが問題ありません。
かなり歳も取ってきて更年期障害の症状が出始めていたので2年前くらいから飲み始めました。最初は指定の量を服用してたのですが私には少し薬の効果が強すぎるせいか副作用がかなり現れたので量を減らして服用していました。それからというもの服用を始める前は息切れや肩こり関節痛がひどかったのですが元気を取り戻し運動もこまめにこなせるようにまで回復しました!
届いたその日に飲みましたが、1回でもけっこう効いてくれました。飲む前はイライラした感じがずっと続いていたのに、少しのことくらいじゃイライラを感じなくなりました。自律神経失調症のために買いましたが、更年期障害の対策としても十分効果が期待できると思います。試しで50錠入りを買いましたが、次回は100錠入りを買ってもいいかなって思います。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。