仕事でのストレスが増えてきた結果、ED気味になっていましたが、これを飲んでみていたらだんだん前のような勃起力を取り戻せるようになってきました。サプリメントでここまで目に見える結果が出るとは思っていなかったですね。ストレス緩和にもよさそうなので、これで効果が期待できそうなうちは飲み続けていきたいと思います。

左記クレジットカード、銀行振込、コンビニ決済に対応

更新日:2025/6/27
スタミナイズRXは、心身の健康をサポートして精力を高め、血行改善によって勃起力を高めることが期待できるサプリメントです。
ペニス増大の効果だけでなく、ストレス緩和など精神的な問題の改善にも役立つとされています。
| メーカー | サファイア・ヘルスケア(Sapphire Healthcare) |
|---|---|
| 主成分 | ナイアシンアミド+L-トリプトファン+アシュワガンダなど |
| 効果 | 総合的な性機能サポート(精力向上・ペニス増大) |
| 副作用 | 報告されていません |
| 用法 | 1日1回、1回分を副用 |
ナイアシンアミドは、優れた抗酸化作用やエネルギー産生作用を持つといわれる成分で、細胞の老化を防ぎ、パワフルな状態を保つことで精力の増強・維持に役立つとされています。
L-トリプトファンは、主に精神的な面からEDにアプローチすると考えられます。精神を安定させて集中力を高めるとされており、性行為への意欲を高めることが期待できます。
アシュワガンダは、アーユルヴェーダ(インドの伝統医学)由来の成分で、心身のストレスを緩和することで精力向上に役立つとされています。

| 個数 | 販売価格(1本あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 1本 | 4,000円 | 4,000円 | 120pt | |
| 3本 | 2,666円 | 8,000円 | 240pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

スタミナイズRX 60錠 x 1本
4,000円
ポイント:120pt
10,000円以上購入で送料無料
在庫あり

仕事でのストレスが増えてきた結果、ED気味になっていましたが、これを飲んでみていたらだんだん前のような勃起力を取り戻せるようになってきました。サプリメントでここまで目に見える結果が出るとは思っていなかったですね。ストレス緩和にもよさそうなので、これで効果が期待できそうなうちは飲み続けていきたいと思います。
ちょっと胃腸の調子がマシになったから、胃腸のケアにはよさそうだった。精力がみなぎっている感覚はあまりない。胃腸が弱い奴にはよさそうだけど、精力アップの効果を高めてほしい。あとサイズアップ効果も。
スタミナイズRXはあくまでサポートツールであり、生活習慣(食事、運動、睡眠)や心理的要因(ストレス管理)も大きな影響を与えます。単体で「完全な解決」を期待するのではなく、健康的な生活などと組み合わせることが重要です。
日常的な疲労感の軽減、集中力向上、スタミナ維持のサポートにも期待できます。特にストレスや加齢によるエネルギーダウンを感じる方には、総合的な活力サポートとして効果が期待されます。
継続して使い続けることが何よりも大切です。サプリメントは医薬品とは違って継続して摂取することでサポート効果があらわれるものです。そのため、短期的な使用で効果を期待することはできません。
摂取を忘れた場合は、忘れたことに気づいたタイミングで摂取するようにしてください。ただし、次の摂取タイミングが近い場合は飛ばしてください。
血流の改善をサポートする成分によって一時的に血圧が変動して、軽い頭重感やめまいを感じている可能性があります。症状が軽い場合は様子を見て、症状がいつまでも続くような場合は医療機関を受診してください。
適切に使用していれば可能性は低いですが、極端に多く摂取したり、長く使っているような場合はバランスが崩れてしまう可能性はあります。
| 1日の摂取回数 | 1回 |
|---|---|
| 1回の摂取量 | 2カプセル |
| 摂取のタイミング | 食事と一緒に |
| 摂取間隔 | 24時間 |
| 商品名 | VP-MAXプラス | ボリュームピルズ | プロソリューション | ビッグRXオイル | マグナRXプラス | メガマックスウルトラ | ハーバルビリリティ |
|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |
| 特徴1 | 複数の成分で精力アップが期待できる | 男らしく逞しい体づくりをサポートする | ・滋養強壮や抗酸化作用のある成分も配合 | 飲むサプリが苦手でも使える外用タイプ | ・様々な男性機能の向上に期待できる | ・男性機能に関する悩みを改善できる | ・10万人以上に愛用されているサプリメント |
| 特徴2 | 体力増進による筋力アップも期待できる | 血流増量によるペニス増大に期待できる | ・勃起力の改善や精子の質改善にも効果的 | 勃起力増強・精液の質向上が期待できる | ・天然成分由来のため安心して服用できる | ・滋養強壮や健康維持に効果的なハーブも配合 | ・勃起不全にも効果的な成分が使われている |
| 内容量 | 60錠 | 60錠 | 60錠 | 60mlx1本 | 60錠 | 60錠 | 90錠 |
| 価格 | 8,450円 | 7,760円 | 7,500円 | 6,300円 | 4,320円 | 7,500円 | 3,150円 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
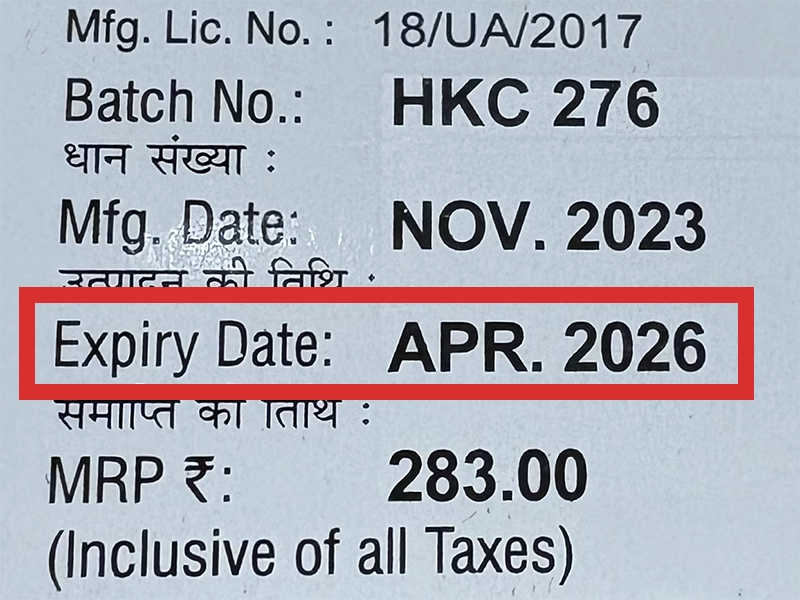
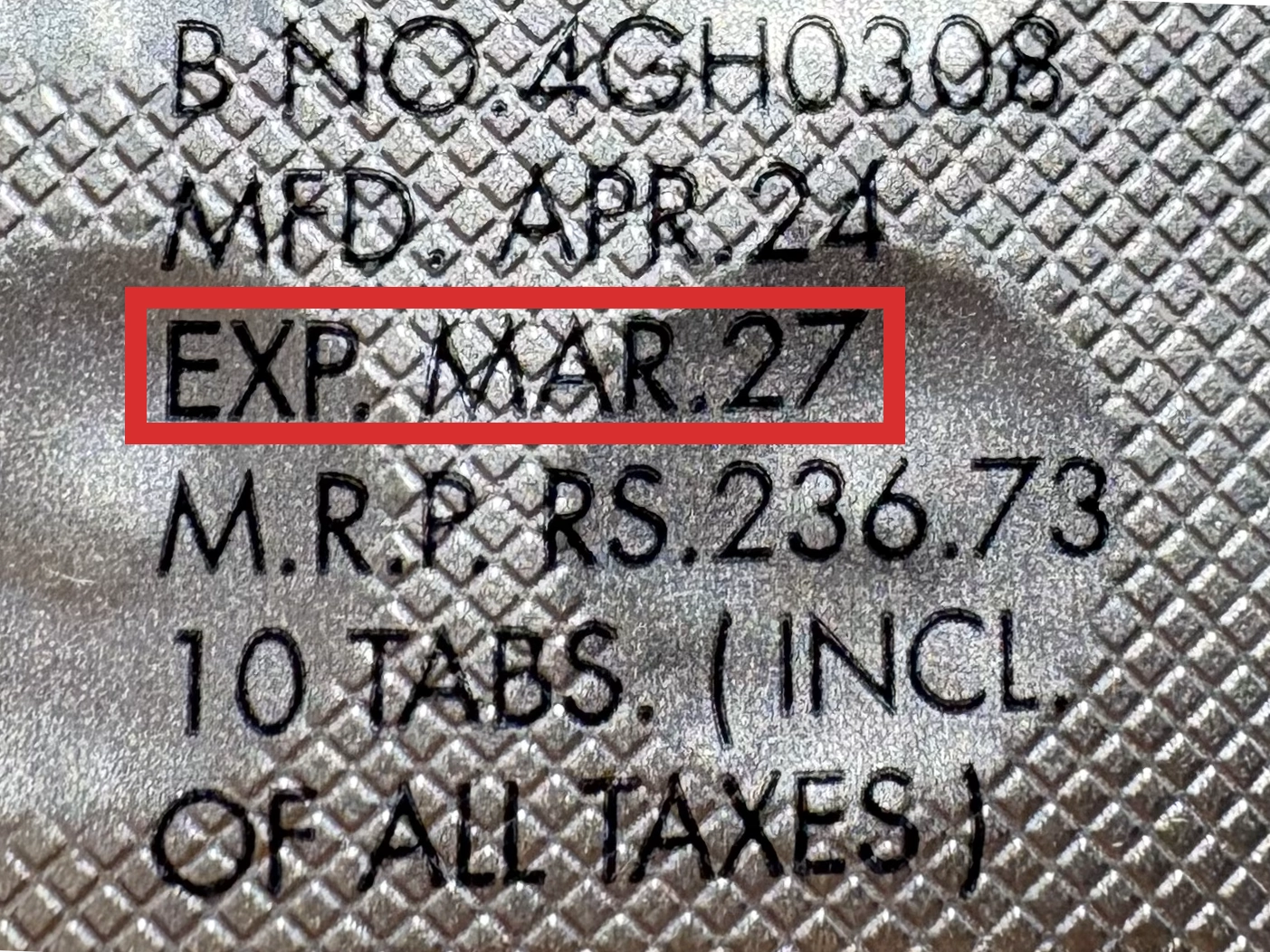
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
仕事でのストレスが増えてきた結果、ED気味になっていましたが、これを飲んでみていたらだんだん前のような勃起力を取り戻せるようになってきました。サプリメントでここまで目に見える結果が出るとは思っていなかったですね。ストレス緩和にもよさそうなので、これで効果が期待できそうなうちは飲み続けていきたいと思います。
とりあえず60錠入りを買ってみて、1日2錠のペースを守って摂取。残りあと1週間分くらいまで使った結果の感想ですが、思っていたよりもよかったです。サプリメントはほとんど効果がないだろうと思っていましたが、前よりも勃起したときの力強さが増したような気がします。次は3本まとめて買ってみようかと考えています。
まだEDではありませんが、EDで悩む人が増えてくる年代に突入したため、ED予防のつもりで飲み始めました。そのおかげか、今のところはEDの症状が出てくることがなく、それどころか前よりも風俗遊びを楽しめるようになりました。嬢の子たちにもデカいと言ってもらえています。アレのデカさで褒められたのははじめての体験でした。
現在、使い始めてから1ヶ月目。目立った変化は感じられないが、精力はじわじわと高まってきた気がする。これは続けてもよさそうだから、このまま継続する。もっと大きな変化が出てきたら、そのときは改めてレビューする予定。
俺の長年のコンプレックスを解消してくれた神サプリ。これを飲み始める前はマージで貧相なムスコだったが、これのおかげでだいぶ逞しいムスコになってくれた。しかも精力も昔より上がった気がする。ムスコの大きさで悩んでいる同志はこれを飲むべき。
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。