ヘビーリピーターです。これで+2cmのサイズアップに成功して以来、コツコツ続けています。これからも続けていくつもりです。下半身に自信を持ちたい同志たちよ!これを手に取るのだ!

左記クレジットカード、銀行振込、コンビニ決済に対応




更新日:2025/4/13

| 個数 | 販売価格(1錠あたり) | 販売価格(箱) | ポイント | 購入 |
|---|---|---|---|---|
| 60錠 | 72円 | 4,320円 | 129pt | |
| 120錠 | 64円 | 7,770円 | 233pt | |
| 180錠 | 60円 | 10,880円 | 326pt |






①1万円以上で送料無料
1回の注文で10,000円以上だった場合、1,000円の送料が無料となります。
まとめ買いをすると1商品あたりのコストパフォーマンスが高くなるためおすすめです。
②プライバシー守る安心梱包
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。

③100%メーカー正規品取り扱い
当サイトの商品は100%メーカー正規品となっており、第三者機関による鑑定も行っております。
商品の破損などがあった場合は再配送などにて対応させて頂きますので、ご連絡頂ければ幸いです。

④いつでも購入可能 処方箋不要
サイト上では24時間いつでもご注文を受けております。
また、お電話によるご注文も受け付けておりますのでネットが苦手な方はお気軽にどうぞ。

⑤商品到着100%
商品発送後はお荷物の追跡状況が分かる追跡番号をご案内させて頂きます。
郵便局には保管期限がありますのでご注意ください。
・自宅配達で不在だった場合の保管期限・・・16日間前後
・郵便局留めとした場合の保管期限・・・7~30日間

⑥コンビニ決済利用可能
ご近所のコンビニにていつでもお支払可能です。
セブンイレブンに限り店舗での機械操作を必要とせず、手続き完了後に表示されるバーコードや払込票番号をレジに提示することでお支払い頂けます。

マグナRXプラス x 60錠
4,320円
ポイント:129pt
10,000円以上購入で送料無料
在庫あり

ヘビーリピーターです。これで+2cmのサイズアップに成功して以来、コツコツ続けています。これからも続けていくつもりです。下半身に自信を持ちたい同志たちよ!これを手に取るのだ!
臭いがキツめで飲みにくいし、頑張って飲み続けてもほとんど効果が出ない。いつかは効果が出るはずだと考えて飲み続けていたが、効果が出てくるよりも先に心が折れた。錠剤も大きすぎて飲み込みにくいし、日本人にはかなり使いにくい。海外の人向き。
| 1日の服用回数 | 1回 |
|---|---|
| 1日の服用量 | 2錠 |
| 服用のタイミング | 指定なし |
| 服用間隔 | 24時間以上 |
| 商品名 | VP-MAXプラス | ボリュームピルズ | プロソリューション | ビッグRXオイル | スタミナイズRX | ペニソール | ビッグRXプラス |
|---|---|---|---|---|---|---|---|
| 商品画像 |  |  |  |  |  |  |  |
| 特徴1 | ・疲労回復や滋養強壮にも効果的 | ・体に負担をかけずに男性機能を改善する | ・滋養強壮や抗酸化作用のある成分も配合 | ・塗るだけで2つの効果を得られるオイル | ・男性の力強さや持続力を高めるサプリメント | ・効果的に男性器を充実させるサプリメント | ・幅広い男性器に関する悩みを改善する |
| 特徴2 | ・EDの症状対策にも高い効果が期待できる | ・安全性が高い天然成分のみを配合している | ・勃起力の改善や精子の質改善にも効果的 | ・男性特有の悩みを効果的に解消できる | ・胃腸やストレスケアの効果も期待できる | ・滋養強壮効果があるハーブも配合されている | ・臨床試験でも効果があると確認されている |
| 内容量 | 60錠 | 60錠 | 60錠 | 60mlx1本 | 60錠x1本 | 10錠 | 60錠x1箱 |
| 価格 | 8,450円 | 7,760円 | 7,500円 | 6,300円 | 4,000円 | 3,640円 | 6,700円 |
本製品は海外製のため、期限表記が日本と異なる場合がございます。
パッケージ裏面や側面、シートなどに以下のような表記がされています。
| EXP | 使用期限 例:EXP 12/2025→2025年12月まで使用可 |
|---|---|
| MFG または MFD | 製造日 例:MFG 03/2023 |
| BEST BEFORE | 品質が最も安定している目安日 |
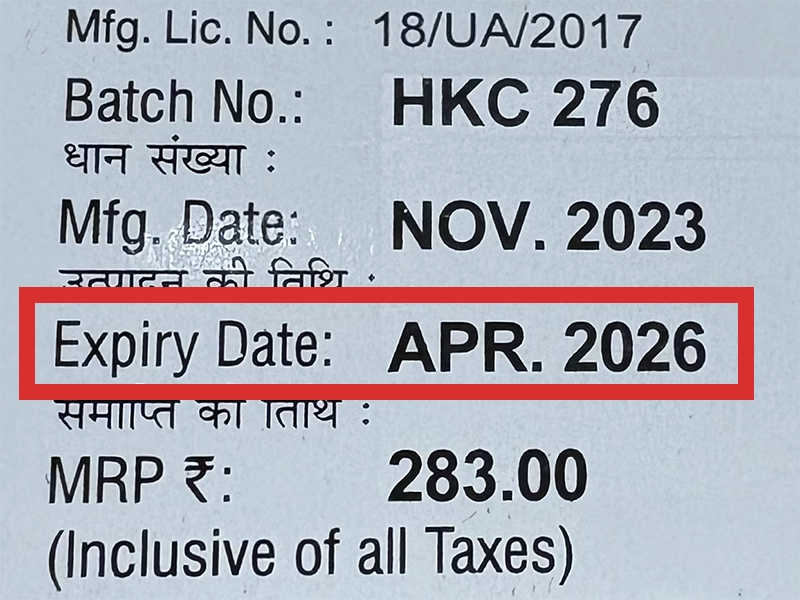
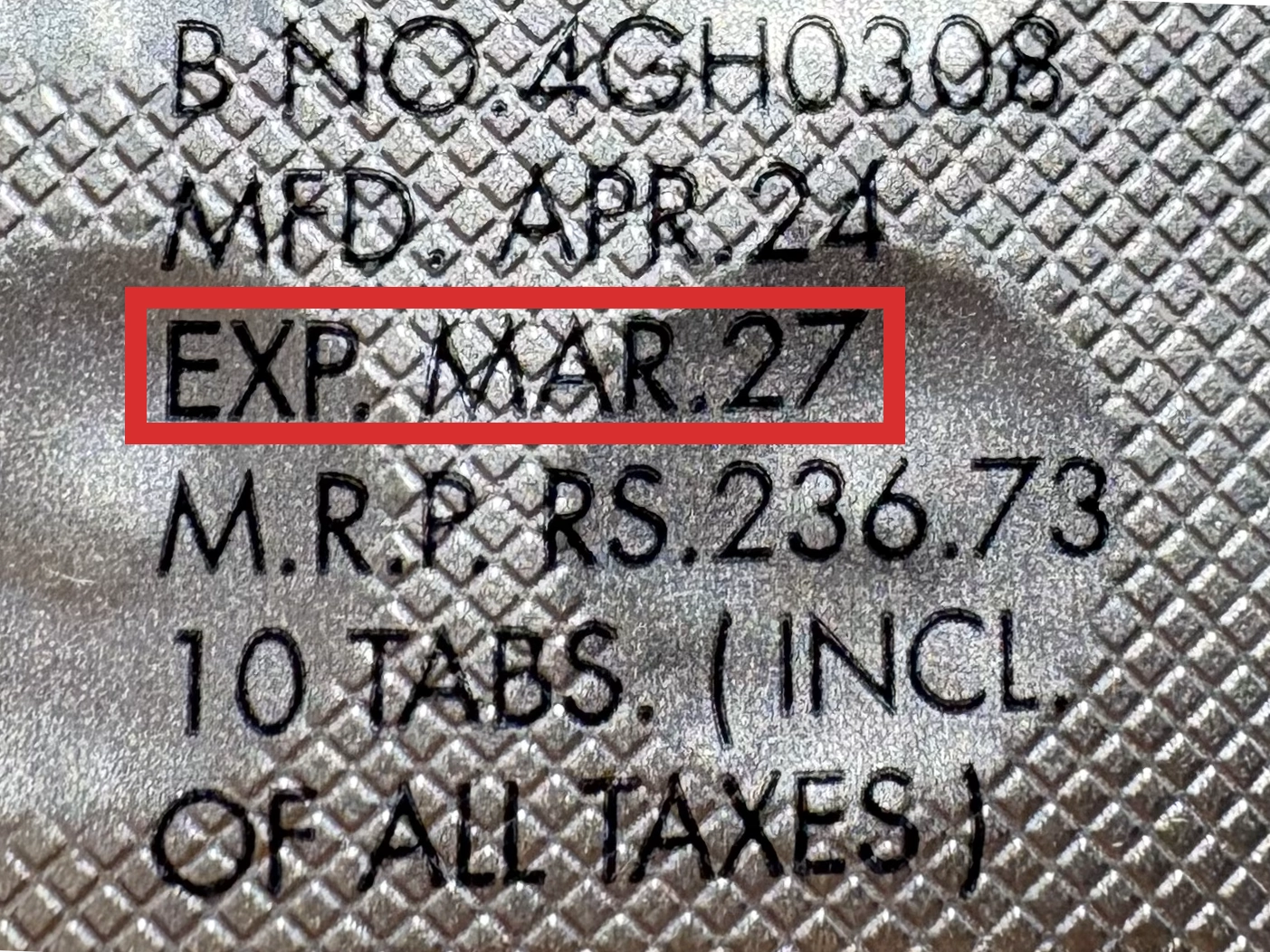
※国や製品により日付の並び(例:月/年、日/月/年)が異なる場合がありますのでご注意ください
EXP(Expiry Date) の表記がなく、MFG または MFDしか記載がないケースがあります。
この場合は MFG(MFD) から2~3年が使用期限の目安です。
※「LOT」や「BATCH」の表記は製造番号であり期限ではありません。

パッケージ例となります。
商品やご注文単位によってはシート単位でのお届けとなる場合が御座います。
外箱に当サイト名や商品名が記載されることはないため、ご家族や配達員など第三者に内容を知られることは御座いません。
匂いと味が気になるが、我慢できないほどではない。長さに変化はみられないが、重さや太さは前よりも上がったような気がする。朝勃ちはほぼ毎日ある。下半身に活力を与えられているのは確かなようだから、もうしばらく様子見。
ヘビーリピーターです。これで+2cmのサイズアップに成功して以来、コツコツ続けています。これからも続けていくつもりです。下半身に自信を持ちたい同志たちよ!これを手に取るのだ!
ムスコの大きさでからかわれたことがあり、それが本当にトラウマで・・・でも、いつまでもトラウマ抱えたままなのも駄目だよなと思って、これを飲んでます。サプリメントだから安心して飲めるし続けやすいし、何より安い!!気長に続けていこうと思います。
この手のサプリはいくつか試したんですが、自分にはこのマグナRXプラスが合ってますね。飲み始めて6ヶ月ほど経過しましたが彼女に「なんか大きくなってない?」と言われました。やったぜ!
若年性のEDっぽいのでまずはサプリからというかんじです。精液の量と濃さが増した気がするけど、気のせいかなあw
商品口コミの投稿は会員のみ行えるようになっております。
お手数ですが会員ログインの上でご投稿頂きますようお願いいたします。
口コミをご投稿頂いたお客様にはポイントをプレゼントさせて頂いております。
文章のみであれば100ポイント、文章+写真付きのものは300ポイントをプレゼントさせて頂きます。
規約や詳細などはこちらをご確認くださいませ。